Developing a personalized template for lymph node dissection during radical prostatectomy
Introduction
Lymph node dissection (LND) remains an integral component of the surgical management of prostate cancer (CaP). In spite of the improvements in imaging techniques, LND is the sole modality that can provide accurate and reliable prognostic information regarding lymph node invasion (LNI) for men with CaP. LND with curative intent is permissible in a subset of patients (1). Moreover, the number of nodes removed during LND has been shown to correlate with time to disease progression (2) and patients (node-negative) with at least 10 nodes removed had a lower risk of cancer-specific death at 10 years compared to patients who did not undergo LND (3). Lastly, in patients with positive lymph nodes, extended LND is associated with improvements in cancer specific survival (4). LND, when performed using the correct technique (5), is not only beneficial but also oncologically necessary in patients with aggressive Ca.
Methods
Several PubMed searches were conducted between October through December 2017 using combinations of the following keywords: lymph node dissection, pelvic, prostate cancer, extended, limited, prostatectomy, fluorescence, PET/MRI, outcomes, and salvage. Appropriate filters were implemented to avoid non-human studies and articles written in English. Moreover, guidelines for LND were abstracted from each organization website [National Comprehensive Cancer Network (NCCN), American Urologic Association (AUA), and European Association of Urology (EAU)].
Papers were selected based on relevance after being matched with the following topics: indications, drainage patterns, number of lymph nodes required for satisfactory dissection, limited vs. extended, and complications. Furthermore, relevant articles regarding the role of LND in various risk groups (AUA risk categories: low-, intermediate- and high-risk) were given importance. References within the above articles were examined and selected as relevant. Review articles, case reports, abstracts, and editorial submissions were removed. The total number or articles included were 31 with additional information abstracted from three organizational guideline statements (NCCN, AUA, EAU).
Review
Indications
During the initial phase of prostate-specific antigen (PSA) testing and CaP screening, a stage migration occurred with more patients presenting with clinically localized disease and without lymph node involvement. Historically, 20% to 40% of patients undergoing radical prostatectomy (RP) and LND were found to be positive (6). However, with this stage migration, only 4–6% of patients undergoing RP had positive nodes at the time of LND (7), prompting some urologists to abandon LND or perform LND in a limited fashion. Contemporaneously, many patients with low-risk, organ-confined CaP are offered active surveillance and conversely patients undergoing RP more commonly require LND at the time of surgery. Unfortunately, indications for LND vary according to the NCCN, AUA, and EAU recommendations (Table 1).

Full table
The NCCN recommends LND in in all patients with a pre-RP nomogram predicting >2% probability of nodal metastasis (8). According the AUA, LND is recommended based on preoperative risk classification where unfavorable intermediate-risk disease and high-risk disease patients undergo LND (9). Lastly, the EAU recommends LND in all patients with high-risk disease and select patients with intermediate-risk disease who have greater than 5% estimated risk of lymph node metastasis (10). Thus, no consensus has been achieved regarding the indications for LND at time of RP.
Drainage patterns
Metastatic nodal CaP invariably encompasses the pelvic lymph nodes but can be extensive and involve para-aortic and para-caval lymph nodes (11). Early scintigraphic mapping studies have identified potential landing zones for CaP in the pelvis and it has been surmised that LND along the obturator fossa and external iliac vasculature comprises approximately 40% of the primary lymph nodes (12). A more extended dissection incorporating lymphatic tissue medial and lateral to the iliac vessels incorporates about two thirds of the primary nodal drainage. Other potential landing spots include the presacral and subaortic nodes (13). Thus, limited template for LND in CaP should include nodes from the obturator fossa and external and internal iliac vasculature.
The frequency of lymph node positivity in these landing zones has been researched. In a contemporary series of 642 consecutive patients with a median of 16 lymph nodes removed, approximately 70% had positive lymph nodes in either the external iliac (11%), obturator fossa (26%), or internal iliac (31%) (14). Relatively few patients (37%) had positive lymph nodes solely confined to the external iliac with a majority of patients having at least 1 positive lymph node in the obturator (60%) and internal iliac (49%) zones (14).
Total number of lymph nodes
A more extensive LND with a greater number of lymph nodes removed has been associated with a higher biochemical recurrence-free survival indicating a possible benefit in removing micro metastatic disease not revealed during histological examination. In a retrospective study of 440 patients, >10 lymph nodes removed increased the lymph node positivity rate from 6% to 15.7% (15). Regardless of approach (open, laparoscopic, or robotic), overall number of lymph nodes removed, the likelihood of lymph node positivity, and complications are similar (16).
Limited vs. extended LND
A limited pelvic LND is confined to the external iliac and cranial to the obturator nerve and has been proven to be insufficient in terms of the detection of nodal metastasis. In fact, LND limited solely to the obturator fossa may miss up to half of the true nodal involvement (2). Extended LND involves removal of all lymph nodes within the external iliac artery and vein, obturator fossa (cranial and caudal), and medial and lateral to the internal iliac artery (11) and should be considered especially in patients with a significant preoperative risk probability of lymph node metastasis. Both the AUA (9) and EAU (10) recommend extended LND.
Complications
Although a greater number of lymph nodes and a more extensive LND is ideal as aforementioned, complications of LND must be considered. The most common complication of LND is lymphocele formation, occurring in up to 60% of cases (17). With an extended LND, as compared to limited, ~3× higher complication rates have been reported with the rate of lymphocele being 10.3% (extended) vs. 4.6% (limited) (18). Rarer intraoperative complications including hemorrhage, ureteral injury, and obturator nerve injury may also occur.
Developing a personalized template for LND in CaP
In developing a personalized template for LND in patients with CaP, indications, drainage patterns, total number of lymph nodes removed, extent, and complications of LND must be considered. PSA, Gleason score, newer imaging modalities, and integrative clinical genomics all play a role in determining the necessity and extent of LND. Moreover, preoperative risk stratification (low, intermediate, or high) can assist in patient selection for LND. Thus, a personalized template should incorporate the aforementioned variables to determine the utility, efficacy, and safety of LND in CaP patients.
Low risk
Consistent with AUA, EAU and NCCN Guidelines, LND in patients with low risk CaP is not indicated. With over 51,000 patients queried in the National Cancer Database, the yield of lymph node positivity was only 0.4%, 10-fold lower than intermediate or high-risk disease (19). Despite the low risk of lymph node positivity, are there any preoperative factors that may select for these patients and thus potentially offer valuable prognostic information? Briganti et al. examined a group of 588 patients with clinically localized CaP and determined that the percentage of positive cores was the most accurate predictor of LNI with an area under the curve of 79.5% (20). Furthermore, genomics has recently been shown to be integral for LNI. After sequencing DNA from 341 patients with CaP, five specific single-nucleotide polymorphisms (SNPs) were found to be major contributors in predicting lymph node positivity increasing the overall predictive accuracy (including clinical factors) by approximately 13% (21). Thus, the need for updated nomograms including percentage of core positivity and genomic analysis is pressing and may influence certain low-risk patients to undergo LND.
Intermediate/high risk
Consistent with the AUA and EAU guidelines, intermediate and high-risk CaP mandate extended LND. Given the likelihood of LNI and the prognostic and possible therapeutic value, LND in this subset of patients is imperative. Although the risk of lymph node positivity in patients with intermediate risk disease is much lower than high risk disease, certain preoperative risk factors in intermediate risk patients can help guide surgeons on the likelihood of LNI. In a series of approximately 1,000 consecutive patients with intermediate risk disease, LNI was more commonly seen in patients with Gleason 7 disease and percentage of positive cores >63% (P<0.001) (22), irrespective of PSA value.
In patients with high risk CaP, performing an extended LND will help target approximately 75% of all landing sites (11). The addition of common iliac and pre-sacral lymph nodes can capture further potential metastatic sites (13) at the expense of a greater risk for complications (18). In a series of approximately 500 high risk patients preoperatively evaluated using the Briganti nomogram, the authors recommended performing a super-extended LND in patients with a risk score ≥30% (13). Thus, LND can be tailored to individual preoperative risk patterns further enhancing a personalized approach to each patient.
Oligometastatic
RP for oligometastatic disease (defined as five or fewer lesions on bone scan and presence or absence of lymphadenopathy) has gained favor with recent reports suggesting a benefit with primary tumor removal. In a series of 11 patients with oligometastatic disease, RP with extended LND (median number nodes removed: 27) resulted in good clinical progression and cancer-specific mortality-free survival rates with a median follow-up of 63 months (23). Thus, in select patients, multi-modal therapy including RP has been shown to be safe and efficacious in the long-term.
Use of newer imaging tools
Newer imaging modalities have evolved and taken centerfold in the diagnosis and management of nodal recurrence in the setting of primary surgical or non-surgical therapy for CaP. PET 11Choline (24), 18Fluciclovine (25), 68Ga-PSMA (26) have expanded the role for salvage lymphadenectomy with acceptable oncologic outcomes. A newer agent, 18F-PSMA-1007, has been utilized to identify radiographic evidence of biochemical recurrence in 12 patients. The authors were able to demonstrate loco-regional recurrence in 3 out 12 patients, lymph node metastasis in 5 out of 12 patients, and bone metastasis in 3 out of 12 patients at exceedingly low PSA values (27). Hybrid PET/MR scanners will improve the sensitivity and specificity of metastatic detection and may ultimately refine LND extend as these scanners come forth to market (28).
Newer tools and techniques
The authors preferred approach is robotically with a supra-umbilical camera port with two flanking robotic ports, and an additional robotic port on the left with a 5 mm and a 12 mm assistant port on the right (29). Moreover, lymph node packets should be sent individually, corresponding to the target zone, i.e., external, internal, obturator, etc. Lastly, the authors would like to contribute two novel concepts in LND in an effort to maximize detection rate and minimize complications—(I) the use of a fully absorbable polymeric clip with an inner polyglyconate track and outer polyglycolic acid body and (II) fluorescence-guided robotic LND (F-GRLND) with indocyanine-green and near-infrared fluorescence (ICG-NIRF).
Utilization of fully absorbable polymeric clip
In order to decrease the rate of lymphocele, meticulous lymphostasis is critical and can be achieved with the use of titanium clips. Unfortunately, significant complications have been associated with the use of common non-absorbable clips including clip migration into the bladder and rectum following RP (30). All of these complications required additional procedures for clip removal with some leading to permanent functional impairment.
A potential solution to these problems is the use of absorbable clips, such as the Lapro-Clip (Covidien, Mansfield, MA, USA). This clip was FDA approved in 1993 and has a strong record of safety and efficacy. The device is a locking polymeric clip consisting of an inner polyglyconate track and outer polyglycolic acid body that is resorbed completely by hydrolysis in 90–180 days (31).
In our series of 100 consecutive patients undergoing RP for CaP, Lapro-Clips were successfully used in all 100 patients without need for further hemostatic or lymphostatic maneuvers. There were 6 misfires that occurred over 1,922 clips for a misfire rate of 0.31%. No evidence of post-operative bleeding such as pelvic hematoma or transfusion requirement, occurred in any patient. No clinically significant or radiographic lymphoceles were detected. No patients suffered untoward complications such as clip migration.
Beyond the issue of migration, absorbable clips decrease radiographic interference on future pelvic imaging studies. Imaging studies of titanium vs. absorbable clips found that at all follow-up time points ranging from 0–6 months after application, radiographic interference on computed tomography was least with absorbable clips. Thus, adequate lymphostasis is imperative in the prevention of lymphocele and can be achieved with a minimal risk of complications utilizing fully absorbable polymeric clips.
F-GRLND with NIRF
NIRF with ICG allows for deeper tissue penetration and can be used as a lymphangiography marker to delineate the anatomical relationships between critical structures and lymph node packets. Properties inherent to ICG that make it useful as a molecular marker include non-toxicity, non-radioactivity and the ability to exhibit NIRF at a wavelength of 800 nm (32). Robotic surgery augmented with fluorescence-guided molecular imaging has shown promise in patients undergoing LND during radical cystectomy (33).
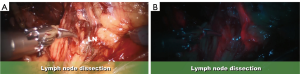
The appeal of LND with fluorescence-guidance and NIRF lies in the detection of sentinel lymphatic drainage and identification of potential clinically involved lymph nodes (Figure 1A,B). Our initial pilot series has shown that F-GRLND with NIRF is safe and feasible and could predict nodal metastasis with approximately 100% sensitivity, 75% specificity, 14% positive predictive value, and 100% negative predictive value (34).
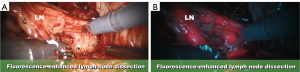
Moreover, F-GLRND with NIRF can accurately “stain” and identify the ureter, obturator nerve, obturator vessels to prevent complications to these vital structures. After direct injection of the prostate with ICG, the sentinel lymph nodes will fluoresce while the ureter, obturator nerve (Figure 2A,B), and the obturator vessels will be a-fluorescent.
Conclusions
LND is a critical component in the management of patients with CaP. Understanding of the indications, drainage patterns, total and extent of LND, utilization of nomograms and genomics, and complication profile can aid in the creation of an individualized template. The use of polymeric clips and F-GRLND with NIRF has the potential to maximize therapeutic benefit and minimize complication risks, ensuring the safety and feasibility of LND in patients with CaP.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bader P, Burkhard F, Markwalder R, et al. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol 2003;169:849-54. [Crossref] [PubMed]
- Bader P, Burkhard F, Markwalder R, et al. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol 2002;168:514-8. [Crossref] [PubMed]
- Joslyn SA, Konety B. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology 2006;68:121-5. [Crossref] [PubMed]
- Abdollah F, Gandgaglia G, Suardi N, et al. More extensive pelvic lymph node disection improves overall survival in patients with node-positive prostate cancer. Eur Urol 2015;67:212-9. [Crossref] [PubMed]
- Menon M, Hemal AK, Team VIP. Vattikuti Institute Prostatectomy. A Technique of Robotic Radical Prostatectomy: Experience in More than 1000 Cases. J Endourol 2004;18:611-9. [Crossref] [PubMed]
- Fowler JE, Whitmore W. The incidence and extent of pelvic lymph node metastases in apparently localized prostatic cancer. Cancer 1981;47:2941-5. [Crossref] [PubMed]
- Petros JA, Catalona W. Lower incidence of unsuspected lymph node metastasis in 521 consecutive patients with clinically localized prostate cancer. J Urol 1992;147:1574-5. [Crossref] [PubMed]
- Mohler JL, Armstrong AJ, Bahnson RR. NCCN Guidelines: Prostate Cancer, Version 3, 2016.
- Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol 2017. [Epub ahead of print]. [PubMed]
- Mottet N, Bellmun J, Briers E, et al. Guidelines on Prostate Cancer. EAU Guidelines, 2015.
- Mattei A, Fuechsel F, Bhatta Dhar N, et al. The template of primary lymphatic landing sites of the prostate should be visited: results of a multimodality mapping study. Eur Urol 2008;53:118-25. [Crossref] [PubMed]
- Klein EA, Kattan M, Stephenson A, et al. How many lymphadenectomies does it take to cure one patient? Eur Urol 2008;53:13-5. [Crossref] [PubMed]
- Gandaglia G, Zaffuto E, Fossati N, et al. Identifying candidates for super-extended staging pelvic lymph node dissection among patients with high-risk prostate cancer. BJU Int 2018;121:421-7. [Crossref] [PubMed]
- Godoy G, von Bodman C, Chade D, et al. Pelvic lymph node dissection for prostate cancer: frequency and distrbution of nodal metastases in a contemporary radical prostatecetomy series. J Urol 2012;187:2082-6. [Crossref] [PubMed]
- van der Poel HG, de Blok W, Tillier C, et al. Robot-assisted laparoscopic prostatectomy: nodal dissection results during the first 440 cases by two surgeons. J Endourol 2012;26:1618-24. [Crossref] [PubMed]
- Ploussard G, Briganti A, de la Taille A, et al. Pelvic lymph node dissection during robot-assisted laparoscopic radical prostatectomy: efficacy, limitations, and complications – a systematic review of the literature. Eur Urol 2014;65:7-16. [Crossref] [PubMed]
- Lee HJ, Kane CJ. How to minimize lymphoceles and treat clinically symptomatic lymphoceles after radical prostatectomy. Curr Urol Rep 2014;15:445. [Crossref] [PubMed]
- Briganti A, Chun F, Salonia A, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol 2006;50:1006-13. [Crossref] [PubMed]
- Modi PK, Bock M, Kim S, et al. Utilization of pelvic lymph node dissection for patients with low-risk prostate cancer treated with robot-assisted laparoscopic prostatectomy. Clinical Genitourinary Cancer 2017;15:e1001-6. [Crossref] [PubMed]
- Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012;61:480-7. [Crossref] [PubMed]
- Oh JJ, Park S, Lee S, et al. A clinicogenetic model to predict lymph node invasion by use of genome-based biomarkers from exome arrays in prostate cancer patients. Korean J Urol 2015;56:109-16. [Crossref] [PubMed]
- Briganti A, Capitanio U, Abdollah F, et al. Assessing the risk of lymph node invasion in patients with intermediate risk prostate cancer treated with extended pelvic lymph node dissection: A novel prediction tool. Prostate 2012;72:499-506. [Crossref] [PubMed]
- Gandaglia G, Fossati N, Stabile A, et al. Radical Prostatectomy in men with oligometastatic prostate cancer: results of a single-institution series with long-term follow-up. Eur Urol 2017;72:289-92. [Crossref] [PubMed]
- Karnes RJ, Murphy C, Bergstralh E, et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11C-choline positron emission tomography/computerized tomography. J Urol 2015;193:111-6. [Crossref] [PubMed]
- Nanni C, Schiavina R, Brunocilla E, et al. 18F-fluciclovine PET/CT for the detection of prostate cancer relapse: A comparison of 11C-Choline PET/CT Clin Nucl Med 2015;40:e386-91. [Crossref] [PubMed]
- Hijazi S, Meller B, Leitsmann C, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography Prostate 2015;75:1934-40. [Crossref] [PubMed]
- Giesel FL, Will L, Kesch C, et al. Biochemical recurrence of prostate cancer: initial results with 18F-PSMA-1007 PET/CT. J Nucl Med 2018;59:632-5. [Crossref] [PubMed]
- Zarzour JG, Galgano S, McConathy J, et al. Lymph node imaging in the initial staging of prostate cancer: An overview and update. World J Radiol 2017;9:389-99. [Crossref] [PubMed]
- Pathak RA, Patel M, Hemal AK. Comprehensive approach to port placement templates for robot-assisted laparoscopic urologic surgeries. J Endourol 2017;31:1269-76. [Crossref] [PubMed]
- Pereira Arias JG, Qunitanilla M, Tamayo A, et al. Complications and incidences in our first 250 robotic radical prostatectomies Actas Urol Esp 2010;34:428-39. [Crossref] [PubMed]
- Hawasli A. The use of absorbable clips in laparoscopic cholecystectomy. J Laparoendosc Surg 1994;4:333-8. [Crossref] [PubMed]
- van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr Opin Urol 2012;22:109-20. [Crossref] [PubMed]
- Manny TB, Hemal AK. Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology 2014;83:824-9. [Crossref] [PubMed]
- Manny TB, Patel M, Hemal AK. Fluorescence-enhanced robotic radical prostatectomy using real-time lymphangiography and tissue marking with percutaneous injection of unconjugated indocyanine green: the initial clinical experience in 50 patients. Eur Urol 2014;65:1162-8. [Crossref] [PubMed]


