Stem cell in urology—are we at the cusp of a new era?
Introduction
The regeneration of the liver of Prometheus may be a myth but the possibility of artificially creating organs has long fascinated humans. Research into stem cells and tissue engineering over the past 2 decades may have in some ways brought us closer to this goal.
Stem cells by definition have characteristics of unlimited expansion with multipotent differentiation capability. While the use of stem cells in modern medicine probably began with the treatment of myeloproliferative disorders with bone marrow transplantation in 1959 (1), much of the research into this has occurred in the last two decades. Renewing or regenerating organ function by tissue repair or formation of new functioning tissue using stem cells, with or without a biological scaffold, became a possibility.
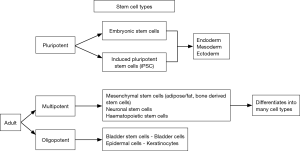
Stem cells (Table 1) are of three major types (2). Human embryonic stem cells derived from 4–5-day-old human embryos. They have high proliferative capability and can differentiate into ectoderm, mesoderm or endoderm. First created in 1998, they raised possibility of an unlimited supply of stem cell lines (3). Harvesting them requires destruction of the human embryo and has raised significant ethical concerns. While the legality of funding research using human embryonic stem cells is now not in question, it is now less commonly used. In a significant breakthrough Takahashi et al in 2006, were able to induce adult differentiated cells to behave like pluripotent stem cells. Described as induced pluripotent stem cells they are similar to embryonic stem cells in terms of morphology and differential potential (4). Despite the breakthrough, doubts remain about the safety of these cells during clinical use. Teratoma formation has been reported when pluripotent stem cells were transplanted in to immunodeficient mice (5). The last types are adult mesenchymal stem cells; derived from hematopoietic and mesenchymal tissues (fat, muscle, skin, brain, etc.). These are multipotent, being capable of differentiation into multiple but not all cell types (Figure 1).

Full table
Tissue engineering is a subset of regenerative medicine that uses biological and engineering principles to construct functional tissues, often using stem cells with or without a scaffold.
Stem cell therapy in urology—current status
An explosion of interest and research in stem cells occurred after the first successful cloning of the sheep, Dolly; by Campbell et al using a somatic cell nuclear transfer (SCNT) technique (6). They transferred a cell nucleus from an adult cell into the unfertilized oocyte of a sheep. Although in the present day, SCNT is unlikely to be used for regenerative medicine in humans, it did spark an interest in stem cell research and allowed for improved access to funding for such research, a trend that has endured.
Urology has always been in the forefront of technological innovation in medicine.
The lower urinary tract is easily accessible by endoscopy with minimal morbidity. The chronic diseases both degenerative and neurological that affect these organs are thought to be excellent candidates for stem cell therapy. For any new and potentially expensive therapy to be viable, three conditions must be fulfilled. First, the prevalence of the disease must be significant. Second, the existing forms of treatment result in suboptimal outcomes and /or low quality of life, and finally, stem cell therapy or tissue engineering must offer potentially greater benefits than existing therapy.
Stress urinary incontinence (SUI)
Assuming that mechanical injury/ dysfunction due to menopause or childbirth to be an important component of SUI, various investigators have injected bone marrow derived, mesenchymal or human amniotic fluid derived stem cells into the sphincter with varying results (7). A preclinical study has shown that both the chronicity of the intrinsic sphincter deficiency and the route of injection of stem cells (local vs. intravenous) affect the outcome with local injection being more effective (8). A randomized clinical trial published in 2007 (9) compared periurethral injections of autologous myoblasts and fibroblasts with conventional collagen in females with stress incontinence. Excellent results were reported at 12 months with 38 of the 42 women completely continent compared to only 2 of the 21 who had received conventional collagen injections. However, investigations by a governmental agency into serious allegations of fraud revealed that the study was not conducted according to Austrian law nor did they follow good clinical practice guidelines. Doubts were raised if such a trial ever existed. The article was later retracted by Lancet (9).
Bladder outlet obstruction
Bladder outlet obstruction causes inflammation, hypertrophy and later fibrosis (10). Reports suggest that mesenchymal stem cells injected into the bladder reduce hypoxia, markers of inflammation with remodeling of collagen in rat models. However the mechanism by which these changes occur has yet to be elucidated completely (11). At the moment, stem cell therapy for this condition remains experimental.
Urethral reconstruction
Attempts have been made to treat urethral stricture with stem cell therapy.
This has been attempted either by engineered grafts (with or without scaffolds) using stem cells or with a tissue engineered autologous tubularized urethra. Raya-Rivera et al. reported on the use of a tubularized urethra, created from stem cells from bladder tissue and seeded on a synthetic tubular meshwork in 5 boys with urethral defects. They reported a 100% success over a median follow up of 71 months (12). These reports however have not been replicated elsewhere. The success of tissue engineered buccal mucosa also has not been comparable to natural buccal mucosa graft in terms of take and success (13).
Bladder augmentation, detrusor underactivity and neurogenic bladder
Both overactive bladder and underactive detrusor have reported to have improved outcomes in preclinical studies with local injections of adipose derived stem cells (14). While there have been case reports in human patients the results have been not been completely successful (15). Bladder augmentation using a tissue engineered bladder has been described in many trials and perhaps is most likely to be adopted clinically (16). The prerequisites of an ideal synthetic bladder material are two-fold. After integration in the host bladder, it should increase the compliance and second, there should be a urinary barrier similar to the urothelium. Most tissue engineered synthetic polymer bladders where it was hoped that there will be ingrowth of bladder cells, fail in this regard. However, in vitro tissue engineering using mesenchymal stem cells seeded on a scaffold or synthetic matrix, which were later implanted in diseased bladders have shown promise, developing characteristics similar to native bladders. Atala et al. described augmentation with a cell seeded scaffold, in a phase 2 clinical study, in 7 patients with myelomeningocele. No post-operative complications were noted and cystogram and urodynamic studies showed increase in bladder capacity and compliance values with a lowering of leak point pressures (17). A more recent study in 10 patients with spina bifida was unable to replicate these results. There was no improvement in the bladder capacity and 40% had serious adverse events with 50% (5/10 patients) requiring reoperative ileocystoplasty (18). Metabolic memory has been hypothesed as a reason for suboptimal results—diseased bladder cells harvested for the production autologous mesenchymal stem cells may produce thick walled poorly compliant tissue. Experimentally bone marrow mesenchymal cells differentiate into urothelial cells when co-cultured with neonatal urothelial cells. This has been seen with human mesenchymal cells and in a mouse model (19). However no clinical human trials have yet been reported. The evidence regarding the long term function and durability of such procedures is awaited and till that time, enterocystoplasty will remain the gold standard.
Erectile dysfunction
In a rat model of post radiation erectile dysfunction, Qiu et al. reported significant improvement in the erectile function after injection of fat derived stem cells (20). Intracavernosal injection of stem cells resulted in their migration in to the major pelvic ganglia in rats with cavernosal nerve injury (21). While this holds promise in post prostatectomy erectile dysfunction, human trials have not yet been reported.
Problems in stem cell therapy today
Any stem cell therapy or tissue engineered product must be better or at the least comparable with the conventional alternative and perhaps more economical. Currently, no existing therapy meets these criteria and it is technology that is in search of an application.
In Urology, as in other surgical fields, the issues of concern are in two broad areas. The first relates to the translation of basic sciences research. Despite more than 2 decades of stem cell research, the only FDA approved treatment using stem cells is bone marrow transplantation for myeloproliferative disorders. The research that is being done in this field and the funding available has grown exponentially over the years, however its translation into clinical practice is minimal. Part of the problem lies in the fact that the exact mechanism of action of stem cells in a particular condition is not exactly known. Proposed mechanisms of action include cell contact, paracrine signaling systems, neovascularization and differentiation to a particular cell type. These cells secrete growth factors and cytokines, which may aid function. The particular environment in which the stem cell finds itself may initiate a specific pathway of differentiation (22). It is often not clear which type of stem cells would be ideal or potentially better for a particular disease apart from the fact that the ideal method of administration of the cells for a particular condition is not always clear.
The initial optimism regarding the rapid translation of stem cell therapy into clinical domain has reduced significantly. For example in stress incontinence—most studies published in the previous decade were phase 2 trials with small number of patients. No large scale clinical trials with randomization have been published recently that offer robust evidence of benefit. Most of the evidence is from case series of small numbers without long term follow up. The only significant randomized trial regarding stem cell therapy in SUI published in 2007 was later retracted (9). The hype regarding stem cell therapy and the pressure to show results has resulted some of the most serious scientific fraud in the history of medical research (23).
The second issue is regulation of stem cell therapy and products. Despite the apparent lack of robust evidence regarding the clinical use of stem cell therapy, there has been a proliferation of stem cell clinics offering poorly vetted dubious therapy. Turner and Knoepfler (24) analyzed the direct to consumer industry celling stem cells in the United States and found 351 US business and 570 clinics marketing stem cell interventions. Most were autologous cell based therapy with 61% being adipose derived, 48% bone marrow derived and 4% were peripheral blood derived stem cell based interventions. Combination stem cell therapy—with a mixture of autologous stem cell types are also advertised. All these are being promoted for a very wide range of clinical indications including urological. The unregulated use of pluripotent or embryonic stem cell products have been documented to cause de novo malignancy. In a case of stem cell tourism (where a patient travels for therapy which may not available or be more expensive in the country of origin), intrathecal injection of combination stem cells resulted in a glial neuroproliferative lesion (25).
In November 2017, the Food and Drug Administration has issued guidelines regarding stem cell use. Stem cells from most sources are now classified as drugs and therefore will be subject to the same scrutiny in this attempt to establish a “clear and modern” framework for regenerative medicine. An exception to this regulation is “same surgical procedure” wherein a procedure where stem cells use is in an autologous manner and is completed in a single surgery. In such a case the stem cells used is not called a biological drug (26). While too little of regulation leads to potentially dangerous therapies, too much of it will dissuade innovation and progress. It is imperative to find a middle path. The FDA expedited programs for regenerative medicine that treats serious conditions offer novel promising therapies fast track designation, accelerated approval and break through therapy designation (27).
The future
Despite challenges, stem cell therapy remains an exciting field. The ability of stem cells to repair diseased tissue is proven. Clinical translation has been slow and yet has been possible, demonstrated most notably by Rama et al. who used limbal stem cells to repair corneal damage in the eye (28). After 2 decades of research, it is apparent that the biological complexity of human organs was initially underestimated. It is not the production of the organ cells alone but of the organ or a part of it with the neural and vascular network that remains the challenge. It is also important to make it in a way that it remains cost effective and better than the conventional therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Thomas ED, Lochte HL Jr, Cannon JH, et al. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest 1959;38:1709-16. [Crossref] [PubMed]
- NIH Stem Cell Information Home Page. In Stem Cell Information [World Wide Web site]. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services, 2016 [cited March 19, 2018]. Available online: //stemcells.nih.gov/glossary.htm
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Knoepfler PS. Deconstructing stem cell tumourogenicity: a road map to safe regenerative medicine. Stem Cells 2009;27:1050-6. [Crossref] [PubMed]
- Campbell KH, McWhir J, Ritchie WA, et al. Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996;380:64-6. [Crossref] [PubMed]
- Vinarov A, Atala A, Yoo J, et al. Cell therapy for stress urinary incontinence: Present-day frontiers. J Tissue Eng Regen Med 2018;12:e1108-21. [Crossref] [PubMed]
- Williams JK, Badlani G, Dean A, et al. Local versus intravenous injections of skeletal muscle precursor cells in nonhuman primates with acute or chronic intrinsic urinary sphincter deficiency. Stem Cell Res Ther 2016;7:147. [Crossref] [PubMed]
- Strasser H, Marksteiner R, Margreiter E, et al. RETRACTED: Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet 2007;369:2179-86. [Crossref] [PubMed]
- Metcalfe PD, Wang J, Jiao H, et al. Bladder outlet obstruction: Progression from inflammation. BJU Int 2010;106:1686-94. [Crossref] [PubMed]
- Woo LL, Tanaka ST, Anumanthan G, et al. Mesenchmal stem cell recruitment and improved bladder function after bladder outlet obstruction: preliminary data. J Urol 2011;185:1132-8. [Crossref] [PubMed]
- Raya-Rivera A, Esquiliano DR, Yoo JJ, et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 2011;377:1175-82. [Crossref] [PubMed]
- Osman NI, Patterson JM, MacNeil S, et al. Long term follow up after tissue engineered buccal mucosa urethroplasty. Eur Urol 2014;66:790-1. [Crossref] [PubMed]
- Nishijima S, Sugaya K, Miyazato M, et al. Restoration of bladder contraction by bone marrow transplantation in rats with underactive bladder. Biomed Res 2007;28:275-80. [Crossref] [PubMed]
- Levanovich PE, Diokno A, Hasneu DL, et al. Intradetrusor injection of adult muscle derieved stem cells for the treatment of underactive bladder: pilot study. Int Urol Nephrol 2015;47:465-7. [Crossref] [PubMed]
- Pokrywczynska M, Gubanska I, Drewa G, et al. Application of bladder acellular matrix in urinary bladder regeneration: the state of the art and future directions . Biomed Res Int 2015;2015. [Crossref] [PubMed]
- Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241-6. [Crossref] [PubMed]
- Joseph DB, Borer JG, De Filippo RE, et al. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty; phase 2 study in children and adolescents with spina bifida. J Urol 2014;191:1389-95. [Crossref] [PubMed]
- Ning J, Li C, Li H, et al. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 2011;63:531-9. [Crossref] [PubMed]
- Qiu X, Villalta J, Ferretti L, et al. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy- induced erectile dysfunction. J Sex Med 2012;9:1834-41. [Crossref] [PubMed]
- Fandel TM, Albersen M, Lin G, et al. Recruitment of intra- cavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol 2012;61:201-10. [Crossref] [PubMed]
- Elnakish MT, Hassan F, Dakhlallah D, et al. Mesenchymal Stem Cells for Cardiac Regeneration: Translation to Bedside Reality. Stem Cells International. 2012;2012. [Crossref] [PubMed]
- van der Heyden MA, van de Ven T, Opthof T. Fraud and misconduct in science: the stem cell seduction: Implications for the peer-review process. Netherlands Heart Journal 2009;17:25-9. [Crossref] [PubMed]
- Turner L, Knoepfler P. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell 2016;19:154-7. [Crossref] [PubMed]
- Berkowitz AL, Miller MB, Mir SA, et al. Glioproliferative Lesion of the Spinal Cord as a Complication of "Stem-Cell Tourism". N Engl J Med 2016;375:196-8. [Crossref] [PubMed]
- Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. (2017). 1st ed. [ebook] Silver Springs, MD: FDA. Available online: [Accessed 1 Dec. 2017].https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM585414.pdf
- Expedited Programs for Regenerative Medicine Therapies for Serious Conditions: Draft Guidance for Industry (2017). 1st ed. Silver Springs, MD: FDA. Accessed on [Accessed 1 Dec. 2017].https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM585414.pdf
- Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55. [Crossref] [PubMed]