Use of secondary contraception following vasectomy: insights from the Pregnancy Risk Assessment Monitoring System, 2007–2011
Introduction
The most effective male contraceptive method is vasectomy, or permanent surgical sterilization, where the vas deferens associated with each testicle is severed and/or ligated to prevent the addition of sperm within the ejaculate. In the United States, vasectomy is three times less common than tubal ligation, despite its lower cost and lower risk of complications (1,2). An estimated 175,000–526,000 vasectomies are performed in the United States annually (3,4); however, unintended pregnancy after vasectomy can occur. Immediately following vasectomy, remaining sperm within the male reproductive tract may still fertilize ova after intercourse and ejaculation (5). In rare circumstances, recanalization of the vas may lead to vasectomy failure (6). Failure rates, specified as the occurrence of unintended pregnancy following vasectomy, range from 0.2–1.5% annually (7-9). Unintended pregnancy and birth is associated with risk of adverse health, psychosocial, and economic outcomes for both mothers and children. Unintended birth is associated with delay in appropriate prenatal care, poor health in childhood, and increased costs to families and taxpayers (10-12).
Currently, the American Urological Association (AUA) recommends that sexually active couples use a second contraceptive method until a non-centrifuged, post-vasectomy semen analysis (PVSA) performed 8–16 weeks after the procedure reveals azoospermia or rare non-motile sperm (RNMS <100,000 non-motile sperm) (9). However, the AUA guidelines do not specify a recommended type of secondary contraceptive method that should be used (9). Reported rates of compliance with PVSA are low and estimated to range from 48–71% (9,13,14). Similarly, use of secondary contraception following vasectomy is also thought to be low; however evidence is limited (15). Having population-based information on the frequency of secondary contraceptive use and type and effectiveness of methods used is important for both urologists performing vasectomies and couples undergoing this procedure, given the risk of unintended pregnancy in the immediate post-vasectomy period. This information would be valuable for designing counseling interventions to improve use of secondary contraception among couples seeking vasectomy.
We assessed the prevalence of secondary contraceptive use among married postpartum women reporting partner vasectomy using data from the Pregnancy Risk Assessment Monitoring System (PRAMS).
Methods
Data from PRAMS were used to assess the prevalence of secondary contraception during the postpartum period among married women reporting partner vasectomy. PRAMS is a state- and population-based surveillance system established in 1987 and managed by the Centers for Disease Control and Prevention (CDC) in collaboration with respective state health departments. PRAMS surveys women 2 to 6 months postpartum after a live birth; details of the PRAMS methodology have been published previously (16). Data were analyzed from 15 sites (Arkansas, Colorado, Michigan, Missouri, North Carolina, Nebraska, New York, Ohio, Oregon, Rhode Island, South Carolina, Tennessee, Utah, West Virginia, and New York City) that participated in PRAMS during 2007–2011. The site inclusion criteria comprised an overall weighted response rate of ≥65% for at least one year during the study period and collection of data on the types of postpartum contraceptive method used.
To assess postpartum use of secondary contraception among married women reporting partner vasectomy, we used the following PRAMS question: “What kind of birth control are you and your husband or partner using now to keep from getting pregnant?” Response options included were “tubes tied or closed; vasectomy; pill; condoms; injection once every 3 months (Depo-Provera®); contraceptive implant (Implanon®); contraceptive patch (OrthoEvra®); diaphragm, cervical cap, or sponge; vaginal ring (NuvaRing®); IUD (including Mirena®); rhythm method or natural family planning; withdrawal (pulling out); and not having sex (abstinence).” This question also allows respondents to write-in other options, which investigators recoded into one of the above categories as appropriate, with the exception of spermicide, which was added as its own category. These analyses were limited to married women whose partner underwent vasectomy to help ensure that the partner who fathered the pregnancy was the same individual who underwent vasectomy. Current evidence from other sources suggest most couples seek vasectomy during pregnancy or immediately after delivery (17,18). By limiting our analyses to those women surveyed within the first 4 months postpartum, we assumed secondary contraception with vasectomy was indicated. This assumption is based on the current recommendation for use of secondary contraception with vasectomy until negative findings on a PVSA 8–16 weeks after vasectomy (9). This restriction was necessary since the exact time of partner vasectomy cannot be determined within PRAMS. Based on contraceptive failure rates during typical use (i.e., inconsistent and/or incorrect use) resulting in unintended pregnancy, reversible contraception considered secondary methods were placed in three tiers: most effective (<1% failure rate), moderately effective (6–12% failure rate), and least effective (≥18% failure rate) (15,19). The most effective reversible methods included intrauterine devices (IUDs) and implants; moderately effective methods included oral contraceptives, injectables, contraceptive patch, vaginal ring, and diaphragm; and least effective methods included condoms, contraceptive sponges, spermicides, rhythm method, and withdrawal (16). Although the diaphragm has been categorized as moderately effective during typical use, for this report, that method was categorized as least effective because the PRAMS question combines diaphragm/cap/sponge as a single response option, making it impossible to determine which method was used (15,19).
A total of 1,123 married participants reported partner vasectomy as their postpartum birth control method. Seventy-four participants who reported both tubal ligation and partner vasectomy were excluded, as use of additional reversible contraception would not be indicated. In addition, we excluded 45 participants who responded that they were not currently having sex, leaving a total of 1,004 participants at risk for unintended pregnancy for analyses.
We calculated the overall percentage of participants who reported not using secondary contraception among those reporting recent partner vasectomy. In addition, for participants reporting use of secondary contraception, we calculated the percentage who used each method by category of effectiveness during typical use. If a respondent indicated using multiple contraceptive methods postpartum in addition to vasectomy, the most effective reversible method was coded as the primary secondary method. Multivariable logistic regression with prevalence odds ratios (POR) and 95% confidence intervals was used to evaluate correlates of use of secondary contraception. We considered the following as independent variables: maternal age, maternal race/ethnicity, maternal education, paternal education, parity, household income, study site, and year of infant birth. With the exception of income, which was self-reported on PRAMS, all variables were obtained from birth certificates. Software for survey data analysis (SUDAAN) was used for analysis to account for PRAMS complex survey design and non-response (16). Statistical significance was determined at P value <0.05. The PRAMS protocol was reviewed and approved by the Institutional Review Board of the CDC.
Results
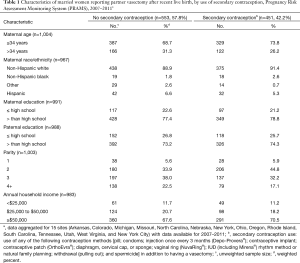
During 2007–2011, a total of 35,525 married women in 15 PRAMS states and New York City had a recent live birth and reported using a postpartum contraceptive method. Of these women, 1,004 married women (3%) reported partner vasectomy as their postpartum contraceptive method. The majority of participants were ≤34 years of age (71%), self-identified as non-Hispanic white (90%), had completed >12 years of education (78%), had a partner who completed >12 years of education (74%), and had an income exceeding $50,000 (69%) (data not shown). Among this cohort, 57.8% reported that they did not use any type of secondary contraception postpartum. There was minimal variation in characteristics between women who reported use of secondary contraception in addition to vasectomy compared with those who did not (Table 1).
Full table
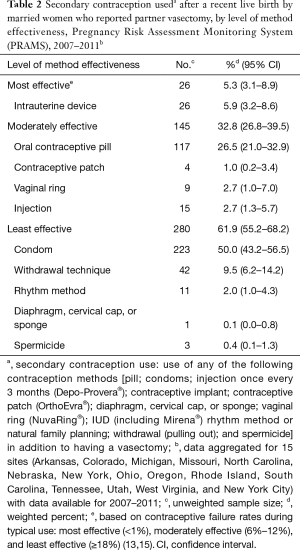
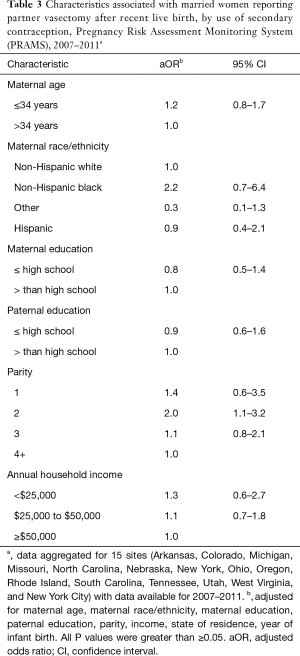
Overall, 5.3% of women reporting a secondary method along with partner vasectomy used highly effective methods; 32.8% used moderately effective methods; and 61.9% used less effective methods. Among participants who reported using a secondary contraceptive method with vasectomy, condoms were most commonly used (50.0%), followed by oral contraceptives (26.5%) and withdrawal (9.5%) (Table 2). Multivariable modeling revealed that use of secondary contraception was twice as high among women reporting a second birth versus women reporting a fourth or higher birth [adjusted odds ratio =2.0 (1.1–3.2)] (Table 3). No other sociodemographic characteristics (maternal age, maternal race, parental education, household income) were significantly associated with use of secondary contraception following vasectomy.
Full table
Full table
Discussion
Nearly 60% of married, postpartum women who participated in PRAMS during 2007–2011 and whose partner had a vasectomy reported not using secondary contraception following their most recent live birth. Furthermore, most women reporting secondary contraception used condoms, which tend to be among the least effective methods unless used consistently and correctly with each act of intercourse (19).
The AUA recommends couples relying on vasectomy use secondary contraception in the period immediately following vasectomy given the risk of pregnancy due to inadequate sperm clearance post-vasectomy or recanalization (9). There are no guidelines from the AUA regarding use of highly or moderately effective methods of secondary contraception prior to PVSA. Issuance of such recommendations could help reduce the likelihood of unintended pregnancy in the immediate period following vasectomy. Long-acting reversible contraception (LARC), such as IUDs or implants, while highly effective, require insertion and removal by a healthcare provider and may not be cost-effective given the short duration following vasectomy during which additional contraception is recommended. However, specific recommendations for continual use of LARC, if initiated prior to partner’s vasectomy until successful PVSA is performed, could be beneficial. Moderately effective contraceptive methods, such as oral contraceptive pills, may be more appropriate methods of secondary contraception for women until their partners’ PVSA verifies azoospermia. These methods offer the advantage of not requiring use with each act of intercourse and provide coverage for the limited time secondary contraception is needed. Less effective methods, such as condoms, could also be recommended by providers. However, counseling for both moderate and less effective methods must emphasize the importance of consistent and correct use to maximize effectiveness during the period immediately following vasectomy.
A primary strength of these analyses is the use of population-based data from women recently giving birth that were collected over a 5-year period. To our knowledge, this is the largest study to systematically examine the use of secondary contraception with vasectomy in the time following a live birth. Prior studies estimating use of secondary contraception following vasectomy are limited. In the Collaborative Review of Sterilization (CREST) study, Jamieson et al. evaluated pregnancy rates in women whose husbands underwent vasectomy and found that among 540 women at risk for pregnancy, 6 pregnancies occurred from 6 to 72 weeks after vasectomy. Two women who became pregnant reported that they did not use secondary contraception (15).
There are also several limitations to this study. PRAMS is cross-sectional in nature, providing a snapshot of a subset of married couples’ decisions regarding postpartum contraception 2 to 4 months following the most recent live birth. Thus, findings may not necessarily reflect either immediate or long-term decisions made by the couple regarding use of secondary contraception in relation to vasectomy. Additionally, the majority of couples identified in this study were well educated, insured, and had income levels greater than the national average, which may limit the generalizability of our results. However, these characteristics are largely representative of couples who undergo vasectomy (20,21). The use of vasectomy, as well as secondary contraception, was self-reported by participants and subject to misreporting and recall bias. Additionally, we were not able to identify alternative methods of postpartum contraception not asked in PRAMS, such or restriction after cesarean section or the lactational amenorrhea method (22-24). Most importantly, we were also unable to evaluate whether a PVSA was performed or whether unintended pregnancy occurred after vasectomy.
The largest limitation of our study was the inability to characterize the precise timing of the partner’s vasectomy (i.e., during pregnancy or after delivery) and relation to the onset secondary contraception use, since this information was not collected in PRAMS. The existing literature is very limited, however, regarding the timing of partner vasectomy for expectant couples. The few small studies that have attempted to characterize the timing of partner vasectomy found the procedure was generally sought during or immediately following the birth of a child (17,18). While we cannot distinguish from these studies the exact proportion of couples who sought partner vasectomy immediately postpartum, our restriction of the PRAMS sample to women who were less than four months postpartum at survey completion provides a reasonable window for estimating the upper bound of the need for use of secondary contraception (4,21).
Nevertheless, to the extent that secondary contraception may not have been needed for couples for whom partner vasectomy occurred more than 8–16 weeks prior to the survey, the risk of pregnancy from not using secondary contraception in this study may be somewhat overestimated. Thus, the proportion of couples we estimate were using secondary contraception represents, at best, the lower limit of overall compliance in this cohort.
Conclusions
With regard to the risk of unintended pregnancy in the immediate period following vasectomy, as many as 57.8% of postpartum women participating in PRAMS who had a recent live birth and reported their partner had a vasectomy did not use secondary contraception with vasectomy. Further, those reporting use of secondary contraception were most likely to use condoms, which are often used inconsistently and/or incorrectly, and among the least effective contraceptive methods. Data from population-based studies focusing on the need for secondary contraception immediately post-vasectomy may be informative in developing effective counseling interventions for providers to offer women and their partners to reduce the likelihood of unintended pregnancy following vasectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The PRAMS protocol was reviewed and approved by the Institutional Review Board of the Centers for Disease Control and Prevention (No. 00-232-4636). Written informed consent was obtained from participants in PRAMS.
References
- Anderson JE, Jamieson DJ, Warner L, et al. Contraceptive sterilization among married adults: national data on who chooses vasectomy and tubal sterilization. Contraception 2012;85:552-7. [Crossref] [PubMed]
- Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23 2005.1-160. [PubMed]
- Barone MA, Hutchinson PL, Johnson CH, et al. Vasectomy in the United States, 2002. J Urol 2006;176:232-6; discussion 236. [Crossref] [PubMed]
- Eisenberg ML, Lipshultz LI. Estimating the number of vasectomies performed annually in the United States: data from the National Survey of Family Growth. J Urol 2010;184:2068-72. [Crossref] [PubMed]
- Bedford JM, Zelikovsky G. Viability of spermatozoa in the human ejaculate after vasectomy. Fertil Steril 1979;32:460-3. [Crossref] [PubMed]
- Schiff J, Chan P, Li PS, et al. Outcome and late failures compared in 4 techniques of microsurgical vasoepididymostomy in 153 consecutive men. J Urol 2005;174:651-5. [Crossref] [PubMed]
- Coward RM, Badhiwala NG, Kovac JR, et al. Impact of the 2012 American Urological Association vasectomy guidelines on post-vasectomy outcomes. J Urol 2014;191:169-74. [Crossref] [PubMed]
- Philp T, Guillebaud J, Budd D. Complications of vasectomy: review of 16,000 patients. Br J Urol 1984;56:745-8. [Crossref] [PubMed]
- Sharlip ID, Belker AM, Honig S, et al. Vasectomy: AUA guideline. J Urol 2012;188:2482-91. [Crossref] [PubMed]
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the well-being of children and families. Washington, DC: National Academy Press, 1995.
- Logan C, Holcombe E, Manlove J, et al. The consequences of unintended childbearing: A white paper. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy. 2007. Available online: https://www.childtrends.org/publications/the-consequences-of-unintended-childbearing-a-white-paper/
- Tsui AO, McDonald-Mosley R, Burke AE. Family planning and the burden of unintended pregnancies. Epidemiol Rev 2010;32:152-74. [Crossref] [PubMed]
- Bieniek JM, Fleming TB, Clark JY. Reduced Postvasectomy Semen Analysis Testing With the Implementation of Special Clearance Parameters. Urology 2015;86:445-9. [Crossref] [PubMed]
- DeRosa R, Lustik MB, Stackhouse DA, et al. Impact of the 2012 American Urological Association vasectomy guidelines on postvasectomy outcomes in a military population. Urology 2015;85:505-10. [Crossref] [PubMed]
- Jamieson DJ, Costello C, Trussell J, et al. The risk of pregnancy after vasectomy. Obstet Gynecol 2004;103:848-50. [Crossref] [PubMed]
- Shulman HB, Gilbert BC, Msphbrenda CG, et al. The Pregnancy Risk Assessment Monitoring System (PRAMS): current methods and evaluation of 2001 response rates. Public Health Rep 2006;121:74-83. [Crossref] [PubMed]
- Bressler J, Landry E, Ward V. Choosing vasectomy: U.S. clients discuss their decisions. AVSC News 1996;34:1, 6.
- Landry EW, Ward V. Beyond acceptability: users' perspectives on contraception. [compiled by] World Health Organization [WHO], Reproductive Health Matters. London, England Reproductive Health Matters 1997.58-67.
- Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Nelson AL, et al. editors. Contraceptive technology. 20th ed. New York: Ardent Media, 2011.
- Barone MA, Johnson CH, Luick MA, et al. Characteristics of men receiving vasectomies in the United States, 1998-1999. Perspect Sex Reprod Health 2004;36:27-33. [Crossref] [PubMed]
- Eisenberg ML, Henderson JT, Amory JK, et al. Racial differences in vasectomy utilization in the United States: data from the national survey of family growth. Urology 2009;74:1020-4. [Crossref] [PubMed]
- Kennedy KI, Visness CM. Contraceptive efficacy of lactational amenorrhoea. Lancet 1992;339:227-30. [Crossref] [PubMed]
- Labbok MH. Postpartum Sexuality and the Lactational Amenorrhea Method for Contraception. Clin Obstet Gynecol 2015;58:915-27. [Crossref] [PubMed]
- Van der Wijden C, Manion C. Lactational amenorrhoea method for family planning. Cochrane Database Syst Rev 2015. [PubMed]


