Effect of a formal oncofertility program on fertility preservation rates—first year experience
Introduction
As cancer treatment and prognosis has dramatically improved over the past decades, the number of cancer survivors and the clinical and scientific interest for their care during survivorship has also increased. Reproduction is an important issue affecting their quality of life (1): many of the cancer treatments available—including radiotherapy, chemotherapy and surgery, as well as the disease itself—can have a significant impact on the patients’ future fertility, either transiently or permanently (2). This has been shown to have repercussions well into adulthood, especially in pediatric or young adult cancer patients, whom likely would not yet have started their families at the time of diagnosis. Even 10 years after diagnosis, patients considered infertility resulting from cancer and its treatment as cause of grief and decreased quality of life (3,4). On the other hand, those patients diagnosed at reproductive age who received counseling from fertility specialists reported higher scores on validated questionnaires regarding quality of life and satisfaction with life, and lower scores relative to long-term feelings of regret (5).
For these reasons, international guidelines from both the American Society of Clinical Oncology (ASCO) (6) and the European Society for Clinical Oncology (7) recommend that physicians discuss the risk of infertility with all patients of reproductive age diagnosed with cancer. This should be addressed as soon as possible in their plan of care, as the success of fertility preservation greatly depends on its application early in the treatment plan—essentially before the start of chemotherapy or radiotherapy (6). Cancer survivors at risk of infertility will also need specialized counseling and guidance as some of them may require use of assisted reproductive technologies (ART) (8).
Despite the consensus on the need and relevance for fertility counseling and preservation for cancer patients, especially in those of pediatric and child-bearing age; studies show it remains vastly underutilized: The National Comprehensive Cancer Network acknowledges fertility preservation as the oncologic service with the lowest rates of prescription and implementation among adolescent and young adult cancer patients (9). In our experience, the reasons for this phenomenon may include practical and logistical difficulties to carry on this process, which is often regarded as a non-priority in the clinical context of urgent need for oncologic treatment and many other acute health issues. Misconceptions about the procedure might also contribute, i.e., the time needed for fertility preservation may place an unacceptable delay on oncologic treatment—while there is evidence that patients referred to fertility preservation actually started treatment sooner than their peers (10).
Instituting standardized, formal fertility preservation programs constitutes a valuable tool to address the reproductive needs of oncologic patients at risk of infertility (11). With the aim of improving the care of cancer patients regarding their fertility counseling and reproductive needs, we initiated a formal male fertility preservation program in June of 2016, in the setting of a tertiary referral healthcare academic institution. This study aims to evaluate the initial outcomes of this formal Oncofertility program, examining sperm banking rates in men newly diagnosed with cancer before and after June 2016.
Methods
A standardized male fertility preservation program was implemented in June 2016. Led by a fellowship-trained urologist specialized in andrology and infertility (R Ramasamy), the program was established to centralize all the services required for fertility preservation for oncologic patients, as far as possible, in a single location. These included: (I) counseling on fertility preservation; (II) an andrology laboratory to perform semen analysis and sperm and testicular tissue cryopreservation; (III) surgical equipment and specialized staff to perform invasive sperm retrieval—either bedside epidydimal sperm aspiration, or surgical testicular biopsy in the operating room. In addition to the actual fertility preservation, the program established a multi-disciplinary network to overcome the logistical challenges that might have been preventing patients from accessing oncofertility services. For this purpose, regular conferences and seminars were carried on by the Department of Urology, Oncology and Radiation Oncology to educate the nurses and physicians involved in the care of oncologic patients on the indications and process of fertility preservation.
To evaluate the effects of these interventions, we performed a retrospective, computer-based chart review between 2011 and 2017. We determined the number of male patients newly-diagnosed with any type of cancer at our institution, during the period of study. We also retrospectively reviewed all men undergoing fertility preservation at our center since the institution of a formal oncofertility program in June 2016. Data on demographic and clinical history, diagnoses, and number and type of samples preserved was collected for those patients. We calculated and compared the rates of sperm banking before the initialization of the fertility preservation program (January 1, 2011 to May 30, 2016) and after the first 15 months of the program (June 1, 2016 to August 17, 2017).
Results
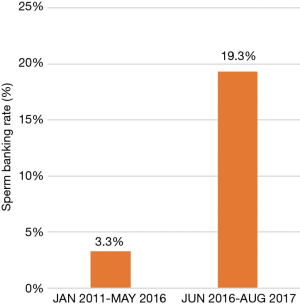
Before a structured oncofertility program was initiated at our institution, 30 of the 902 (3.3%) newly-diagnosed male oncologic patients underwent sperm banking prior to the start of their cancer treatments, from 2011 to May 2016. After implementation of the program in June, 2016, and until August 2017, 42 of 218 newly diagnosed oncologic male patients underwent fertility preservation (19.3%). This represented a 5.8-fold increase in the rate of fertility preservation among male oncologic patients at our institution (Figure 1).
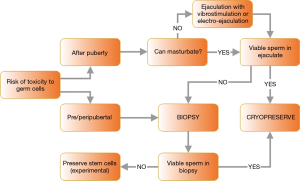
For the patients undergoing fertility preservation after June 2016, their mean age was 30.6±13.1 years (range, 13–69 years), with 6 of them being pediatric patients under 18 years of age 36 of the samples (85.7%) were obtained from masturbation. The 6 remaining patients were not able to provide viable sperm from ejaculation and underwent either testicular biopsies (5 cases) or epididymal sperm extraction (1 case). The sperm retrieval algorithm used in our practice is illustrated in Figure 2 [adapted from Tournaye et al. (12)].
In addition to men undergoing fertility preservation related to oncologic treatment, 56 men also used this service during the period of study, accounting for an overall use of the service of 98 men. Amongst those 56, the most common indications for fertility preservation were: prior to surgical treatment of varicocele (n=17) or vasectomy (n=6); or hypogonadism (n=6).
Discussion
In this present study, we assessed the rates of fertility preservation before and after initiation of a formal male oncofertility program in a tertiary referral academic institution. We found a nearly 6-fold increase in fertility preservation among male oncologic patients after the program was initiated (3.3% to 19.3%). This increase demonstrates its significant impact at our institution, and further indicates that oncofertility programs may provide an excellent tool to address the reproductive needs of oncologic patients resulting in their improved care and quality of life.
The term oncofertility was first used in 2006. The term refers to the field of research and clinical care specifically addressing the future or present reproductive needs of cancer patients, as an interdisciplinary collaboration of oncologists and reproduction specialists (13). Despite great strides made during the last decade, practitioners often do not have the tools or evidence needed to provide individualized fertility counseling to every cancer patient. For example, there is limited evidence to assess with precision the risk of infertility at which each patient is placed by his particular cancer and treatment plan. Risk may vary depending on the type of cancer, the treatment regime, and age and fertility status of the patient at time of diagnosis (14). Additionally, antineoplastic medications are continually being introduced. In a given patient, assessment of infertility risk with older agents may not necessarily predict risk with newer agents (15). For this reason, it is currently agreed by international oncologic societies that the risk of infertility should be disclosed to every cancer patient of reproductive age as early as possible after diagnosis. Counseling on fertility preservation and referral to a specialist should be offered if the patient is interested. ASCO guidelines currently recommend fertility preservation though cryopreservation of sperm for males, and cryopreservation of embryos or oocytes for females, as standard practice for oncologic patients (6). Other methods are still considered experimental; however, evidence indicates their potential for future cases of fertility preservation. These methods include testicular sperm banking, cryopreservation and transplant of autologous testicular tissue, and maturation in vitro of spermatogonial stem cells (12). However, the vast majority of male patients seen in our practice were able to provide sperm by masturbation.
Despite the progress of the field, the main challenge of oncofertility still remains the under-utilization of fertility preservation by target patients (9). Qualitative studies have been performed among experts in the field of fertility preservation, on how to enhance the effectiveness and utilization of oncofertility services. Identified themes include communication between oncology and infertility specialists; management or urgency, management of patients’ expectations, establishment and implementation of standardized protocols, systems and databases; and maintaining contact with patients (16). Following previously validated multidisciplinary approaches (17), our program aims to address each of those themes through incorporating a number of personnel specifically trained to address oncofertility (summarized in Table 1), and emphasizing on the establishment of easy, direct ways of communication between the different members of the team—usually performed telephonically during the established weekly hours of functioning of the fertility preservation service. More importantly, the regular schedule of educational conferences and seminars in the adult and pediatric oncology departments serves two key functions in educating healthcare professionals involved in the care of cancer patients. These conferences (I) repeatedly emphasize the importance of fertility counseling, and (II) continually update information regarding oncofertility services available at our institution. Our program is mostly based on physician-led counseling, but this role has been adopted by nurses in other institutions, with similarly satisfactory results (11).

Full table
The cost of fertility preservation is another well-known challenge to accessibility of oncofertility care. The government of Quebec, Canada introduced coverage for ART in 2010. Early studies evaluating the effect of this measure showed a significant increase in the number of non-cancer male patients undergoing sperm cryopreservation. While the number of cancer patients undergoing fertility preservation appear unchanged, the total number of cryopreservation sessions per patient increased—unlike that found in non-cancer patients (18). This finding strongly suggests that a reduction in costs, or an improvement in insurance coverage of fertility preservation, would have a positive impact on the care of oncologic patients.
Conclusions
In conclusion, the sperm banking rate at our institution increased nearly six-fold among newly diagnosed male cancer patients within a year of the implementation of a formal fertility preservation program. Oncofertility constitutes a relevant part of counseling of cancer patients prior to cancer treatment, and may have a long-term impact on their quality of life. A formal fertility preservation program has the potential to greatly increase rates of sperm banking among cancer patients, and constitutes a relevant clinical need at academic institutions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of the University of Miami and written informed consent was obtained from all patients.
References
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Wasilewski-Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J Cancer Surviv 2014;8:437-47. [Crossref] [PubMed]
- Loscalzo MJ, Clark KL. The psychosocial context of cancer-related infertility. Cancer Treat Res 2007;138:180-90. [Crossref] [PubMed]
- Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol 2005;23:766-73. [Crossref] [PubMed]
- Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012;118:1710-7. [Crossref] [PubMed]
- Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2500-10. [Crossref] [PubMed]
- Peccatori FA, Azim HA Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi160-70. [Crossref] [PubMed]
- Anderson RA, Mitchell RT, Kelsey TW, et al. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556-67. [Crossref] [PubMed]
- Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2012;10:1112-50. [Crossref] [PubMed]
- Goodman LR, Balthazar U, Kim J, et al. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum Reprod 2012;27:2076-81. [Crossref] [PubMed]
- Rotker K, Vigneswaran H, Omil-Lima D, et al. Efficacy of Standardized Nursing Fertility Counseling on Sperm Banking Rates in Cancer Patients. Urology 2017;104:90-6. [Crossref] [PubMed]
- Tournaye H, Dohle GR, Barratt CL. Fertility preservation in men with cancer. Lancet 2014;384:1295-301. [Crossref] [PubMed]
- Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res 2007;138:3-11. [Crossref] [PubMed]
- Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2013;14:873-81. [Crossref] [PubMed]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr 2005.12-7. [Crossref] [PubMed]
- Hammarberg K, Kirkman M, Stern C, et al. Cryopreservation of reproductive material before cancer treatment: a qualitative study of health care professionals’ views about ways to enhance clinical care. BMC Health Serv Res 2017;17:343. [Crossref] [PubMed]
- Johnson RH, Kroon L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Canc Netw 2013;11:71-7. [Crossref] [PubMed]
- Herrero MB, García A, Buckett W, et al. Quebec public funding facilitates fertility preservation for male cancer patients. Curr Oncol 2016;23:20-5. [Crossref] [PubMed]