Fluciclovine positron emission tomography in the setting of biochemical recurrence following local therapy of prostate cancer
Introduction
Prostate cancer (PCa) is the most common non-cutaneous malignancy affecting men in the United States, and in 2017 alone, there was an estimated 161,000 new diagnoses (1). For men with intermediate-risk or high-risk, clinically localized disease, radical prostatectomy or whole-gland radiation therapy-based interventions are pursued as modes of primary definitive treatment with curative intent. However, disease recurrence as measured by a persistence or post-treatment rise in the serum prostate specific antigen (PSA) is not uncommon and occurs in up to 20–30% of men who underwent radical, whole-gland interventions with curative intent (2). For these patients, use of advanced functional imaging modalities such as positron emission tomography (PET) imaging to detect localized intraglandular and extraprostatic sites of persistence or recurrence of disease has become more commonly available and implemented (3-5). Unfortunately, factors such as the generally high metabolic activity of benign prostatic tissue, occasionally low metabolic profile of hormone-naive PCa cells, postoperative scarring and urinary excretion of radiotracers make the delineation of benign from cancerous tissue using traditional radiolabeled glucose analogues challenging (3,6,7).
In May 2016, the Food and Drug Administration (FDA) granted approval for the use of a PET radiotracer, trans-1-Amino-3-[18F]fluoro-cyclobutane carboxylic acid or 18F-Fluciclovine (commercially marketed with the trade name of Axumin by Blue Earth Diagnostics, Oxford, UK), to detect and localize PCa recurrence in patients with a rising PSA following prior therapy (8). 18F-fluciclovine is a synthetic amino acid that is transported by multiple sodium-dependent and sodium-independent channels found to be upregulated in PCa cells (9,10). In addition, 18F-fluciclovine exhibits low urinary excretion which theoretically minimizes background noise when evaluating the prostate bed and surrounding structures including the bladder and urethra (11). It is generally well-tolerated in patients and has a feasible learning curve for interpreting radiologists in this setting (12-14). In a study of 596 men with biochemical recurrence (BCR), no serious adverse events related to fluciclovine were observed (12). Furthermore, repeat administration (n=55) or extravasation of fluciclovine (n=9) did not result in any clinical sequelae (12). Suzuki et al. similarly reported no serious adverse events in a study population of 68 men (13).
Within the past decade, a multitude of studies have evaluated the utility of 18F-fluciclovine PET imaging in the setting of BCR following local therapy for PCa. We performed a systematic review of recently published literature to describe the emerging evidence in support of this imaging modality in comparison to other available staging techniques.
Methods
An English literature search was conducted on PubMed for original investigations on 18F-fluciclovine PET imaging for PCa. Our Boolean search criteria included the following terms: prostate, fluciclovine, FACBC, and Axumin. We excluded publication types such as commentaries, editorials, letters to the editor, guideline statements, reviews articles, or interview transcripts. All remaining articles meeting criteria where18F-fluciclovine PET imaging was used specifically for the detection of PCa in the setting of disease persistence or recurrence after primary therapy with definitive, curative intent were considered. Additionally, the bibliographies of the identified studies were used to expand our search to capture any other relevant articles fitting of the inclusion criteria by content.
Results
Articles reviewed
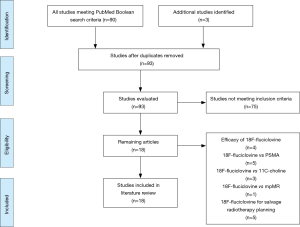
Our review of the literature identified 93 articles. Among these, ultimately, 18 met our inclusion criteria. Four studies evaluated the efficacy of 18F-fluiclovine PET imaging alone in the setting of BCR. Five investigations compared 18F-fluciclovine to various prostate-specific membrane antigen (PSMA)-targeting radiotracers for PET imaging. Four studies compared 18F-fluciclovine to 11C-choline PET imaging, one study compared 18F-fluciclovine to multiparametric magnetic resonance (mpMR) imaging, and we also identified five additional investigations assessing its role in guiding salvage radiation therapy planning (Figure 1) (15).
Diagnostic efficacy of 18F fluciclovine PET imaging
For patients who underwent surgical, radiation-based, or multimodal intervention as primary treatment for PCa, 18F-fluciclovine PET imaging appears to offer a reliable detection rate for both locally persistent disease and extraprostatic disease persistence versus recurrence. Compared to computed tomography (CT) alone, 18F-fluciclovine PET/CT imaging provided a superior sensitivity and specificity for disease recurrence in a 43 patient cohort with histologically confirmed BCR (16). In a retrospective multi-institutional analysis where 143 subjects with previous primary treatment underwent 18F-fluciclovine PET/CT imaging in the setting of BCR, the positive predictive value for local and extraprostatic disease was 71.8% and 92.3%, respectively, compared to a gold-standard of truth based upon histological evaluation (12). In a retrospective cohort analysis of 26 men who underwent 18F-fluciclovine PET/CT imaging, Kairemo et al. also observed a reliable cancer detection rate in this setting. Image positivity was significantly associated with a short PSA doubling time, but not with PSA value alone (17).
Uptake of 18F-fluciclovine also appears to remain stable and reproducible over time in lesions suspected to represent metastatic disease foci. In a retrospective cohort of 14 patients with BCR who underwent two 18F-fluciclovine PET/CT scans serially over a mean interval of 17 months without receiving any new or added forms of therapy based on the initial PET scan findings, there was no significant change in the degree of uptake of previously appreciated within the metastatic lesions compared to background structures (18).
18F-fluciclovine versus PSMA-targeted PET imaging
Our review identified two studies comparing the diagnostic efficacy of 18F-fluciclovine to the PSMA monoclonal antibody, 111-In-capromab (ProstaScint). Schuster et al. prospectively evaluated 50 men with clinical evidence of BCR following primary local therapy by performing both 18F-fluciclovine and ProstaScint PET/CT within 6 weeks of each other. Using clinical information such as PSA nadir following salvage therapy and available confirmatory pathology as a reference standard, 18F-fluciclovine appeared to outperform 111-In-capromab in this setting. 18F-fluciclovine PET imaging demonstrated a more optimal sensitivity (89% vs. 69%) and specificity (67% vs. 58%) profile for prostatic bed recurrence. A similar pattern was observed for extraprostatic recurrence (sensitivity of 100% vs. 10%, specificity of 100% vs. 47%) (19). Similar conclusions were reached in a subsequent prospective analysis of 93 men with BCR. 18F-fluciclovine PET exhibited superior sensitivity to ProstaScint in the detection of both prostatic bed and extraprostatic PCa recurrence (90.2% vs. 67.2%, P=0.002 and 55.0% vs. 10.0%, P<0.001), but no statistically significant difference in terms of diagnostic specificity was observed (20).
We found two studies comparing 18F-fluiclovine to a novel PSMA-targeting radiotracer, 68Ga-PSMA-1. In a prospective cohort of 288 men with BCR where 68Ga-PSMA-1 PET/CT was being investigated, Calais et al. identified 10 patients who were found to have previously undergone 18F-fluciclovine PET scan imaging. In this subgroup, 18F-fluciclovine underperformed 68Ga-PSMA-1 in the detection of suspicious metastatic foci (2 vs. 7 positive scans) (21). The retrospective nature and small cohort in this study represent several limitations. Additionally, metastatic disease progression in the interval between the 18F-fluciclovine PET and 68Ga-PSMA-1 PET imaging could not be ruled out. However, a separate case report also describes a patient who underwent radical prostatectomy and salvage radiation therapy with subsequent BCR who had an unremarkable 18F-fluciclovine scan but significant uptake in a pelvic lymph node on Ga-PSMA PET/CT (22). Thus, a prospective trial is currently underway to compare these imaging agents in this clinical setting (23).
One case report compared a different PSMA-targeting radiotracer, 18F-DCFPyL, to 18F-fluciclovine. In this report a 79-year-old male with clinical evidence of BCR underwent both 18F-DCPyL and 18F-fluciclovine PET/CT. While radiotracer uptake at an area of extraprostatic recurrence was appreciated in both studies, the PSMA radiotracer exhibited superior lesion conspicuity but with greater background noise compared to the 18F-fluciclovine study due to very low levels of 18F-fluiclovine urinary excretion through the nearby ureters and urinary bladder (24).
18F-fluciclovine versus 11C-choline PET imaging
We identified three studies comparing the diagnostic utility of 18F-fluciclovine to 11C-choline PET imaging. In a prospective study by Nanni et al., 28 men with clinical BCR following radical prostatectomy underwent both 18F-fluciclovine PET imaging and 11C-choline PET within a 1-week period. Patient-based and lesion-based analyses of the two imaging modalities resulted in 18F-fluciclovine having a greater sensitivity and specificity for the detection of local and extraprostatic sites of disease persistence versus recurrence (25). A subsequent prospective analysis of 89 men with BCR following radical prostatectomy revealed similar findings; compared to 11C-choline PET, 18F-fluciclovine demonstrated a superior sensitivity, specificity, and positive predictive value for PCa recurrence using a reference standard of 1-year follow up, PSA trend, and pathology when available (26). The comparative advantage of 18F-fluciclovine PET imaging was appreciated in a salvage pelvic lymph node dissection for PCa. The patient in this report underwent both 11C-choline and 18F-fluciclovine PET evaluations preoperatively. The 11C-choline study was nondiagnostic whereas 18F-fluciclovine revealed two suspicious pelvic lymph nodes which were later found to be positive for metastatic spread of PCa to those lymph nodes on final pathology (27).
18F-fluciclovine PET versus mpMR imaging
One recently published study compared the detection rate and diagnostic accuracy of 18F-fluciclovine PET to mpMR imaging. In a cohort of 24 men who underwent nonsurgical primary therapy who exhibited clinical BCR, 18F-fluciclovine PET and mpMR imaging were performed within a 29-day time period. The disease detection rate in this cohort was 94.7% using 18F-fluciclovine PET compared to 31.6–36.8% for mpMR imaging using a multidisciplinary truth panel as a reference standard. In addition, 18F-fluciclovine PET exhibited less inter-observer variability than mpMR imaging (weighted kappa of 0.9 and 1 for local and extraprostatic disease vs. 0.25 and 0.74) (28).
Utility of 18F-fluciclovine PET imaging for salvage radiation therapy planning
Multiple studies evaluated the utility of 18F-fluciclovine PET imaging in the setting of post-prostatectomy salvage radiation therapy treatment planning. Brunocilla et al. were the first to report a case of clinically successful salvage radiotherapy in a patient who underwent 18F-fluciclovine imaging in the setting of BCR after prostatectomy where recurrent disease was identified in the prostatic fossa (29). Of note, the patient also underwent mpMR and 11C-choline PET imaging which both failed to identify recurrent disease. Extrapolating from a randomized prospective clinical trial of patients with BCR who were scheduled to undergo salvage radiotherapy, Schreibmann et al. reported that 30 of 41 men who underwent pre-treatment 18F-fluciclovine PET had alterations made to their planned radiotherapy target volumes (30). Two subsequent prospective analyses of 87 and 96 men reported significant augmentations to salvage radiation therapy dosing plans when pre-treatment 18F-fluiclcovine PET imaging was utilized (31,32). In all three studies, both the dosing amount and anatomical regions of focus were significantly impacted by the information provided by 18F-fluciclovine PET imaging.
Discussion
In men who undergo treatment for localized PCa, at least one in five will exhibit some form of disease recurrence defined as BCR by means of serum PSA follow-up assessments (2). In this setting, numerous diagnostic imaging modalities exist to identify the location and extent of recurrence. 18F-fluciclovine PET was recently granted FDA approval for this purpose (8). Our aim was to summarize recent evidence evaluating the diagnostic utility of this imaging modality and to compare it to other available radiotracers.
In one of the largest studies evaluating the role of 18F-fluciclovine PET in patients with clinical evidence of BCR following local therapy for PCa, Bach-Gansmo et al. observed that this modality offers a reliable positive predictive value for detection of both local and extraprostatic disease recurrences (12). Kairemo et al. also reached similar conclusions in a smaller cohort (17). Furthermore, radiotracer uptake in both metastatic lesions and background tissue appears to remain stable over time, so providers could utilize this modality with confidence in the interpretation of the study results at any time point in the patient follow-up algorithm when PCa recurrence is suspected, though correlation with heightened sensitivity has been associated with higher levels of serum PSA in the post-treatment setting (18).
Conversely, there is a relative paucity of data evaluating the diagnostic accuracy of 18F-fluciclovine PET imaging in the setting of recurrent disease following various secondary interventions including salvage radiation or surgical interventions. Theoretically, the use of PET imaging should be equally valuable in these setting as sites of nodal or soft tissue involvement with PCa outside the operative field or irradiated space should be unaffected and hence physiologically equivalent for imaging with these radiotracers. Further investigation in this clinical setting as well as in the case of disease persistence or progression following androgen deprivation therapy, chemotherapy, immunotherapy, or other systemic therapy is critical in determining the clinical value of these PET imaging modalities after systemic treatments and potential clonal selection for subclones of PCa able to survive and progress through these treatment approaches.
Multiple PET radiotracers are available to assess disease burden in the setting of PCa BCR in addition to 18F-fluciclovine, namely an assortment of PSMA-targeting radiotracers and 11C-choline. Compared to ProstaScint, two prospective trials observed superior sensitivity and often specificity of 18F-fluciclovine for the detection of recurrent PCa foci (19,20). However, 18F-fluciclovine was inferior to 68Ga-PSMA PET in the detection of recurrent disease foci albeit in a small retrospective cohort and with comparison to only one of several actively investigated PSMA-targeting PET tracers (21). When compared alongside 11C-choline PET imaging, 18F-fluciclovine demonstrated superior recurrent cancer detection in two prospective analyses (25,26). While promising, the relatively small study population sizes, lack of consistent histological confirmation of recurrent disease foci detected on imaging, and novelty of these imaging modalities warrant further scrutiny in larger cohorts with long-term follow-up.
Beyond disease detection, we encountered several studies evaluating the role of 18F-fluciclovine PET imaging in guiding and mapping salvage external beam radiation therapy (30-32). All of these studies found that pre-radiotherapy 18F-fluciclovine PET augmented the location and intensity of administered radiation treatments. Furthermore, cases typically treated with salvage pelvic radiation with a distant focal metastatic site defined by PET imaging may obviate the potential side effects and complications of salvage radiation therapy to a presumed pelvic bed while allowing a more stereotactic approach to the “out-of-field” focus of metastatic spread versus systemic therapy for multifocal extra-template sites if present. Additional studies are warranted to elucidate whether these dosing changes had any impact on oncologic outcome.
Conclusions
In the setting of BCR following local therapy with definitive, curative intent for PCa, 18F-fluciclovine PET imaging is a safe and well-tolerated imaging modality with optimized diagnostic yield over standard staging imaging with CT and nuclear medicine bone scan. Further investigations comprising larger populations undergoing these PET imaging studies are warranted to support the early findings as its use expands in clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: S Rais-Bahrami serves as a consultant for Blue Earth Diagnostics and Philips/InVivo. The other author has no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Martino P, Scattoni V, Galosi AB, et al. Role of imaging and biopsy to assess local recurrence after definitive treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU). World J Urol 2011;29:595-605. [Crossref] [PubMed]
- Picchio M, Messa C, Landoni C, et al. Value of [11C]choline-positron emission tomography for re-staging prostate cancer: a comparison with [18F]fluorodeoxyglucose-positron emission tomography. J Urol 2003;169:1337-40. [Crossref] [PubMed]
- Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol 2011;59:51-60. [Crossref] [PubMed]
- Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Semin Nucl Med 2016;46:502-6. [Crossref] [PubMed]
- Rais-Bahrami S, Pietryga JA, Nix JW. Contemporary role of advanced imaging for bladder cancer staging. Urol Oncol 2016;34:124-33. [Crossref] [PubMed]
- Parent EE, Schuster DM. Update on (18)F-fluciclovine PET for prostate cancer imaging. J Nucl Med 2018;59:733-9. [Crossref] [PubMed]
- Okudaira H, Oka S, Ono M, et al. Accumulation of trans-1-amino-3-[(18)F]fluorocyclobutanecarboxylic acid in prostate cancer due to androgen-induced expression of amino acid transporters. Mol Imaging Biol 2014;16:756-64. [Crossref] [PubMed]
- Ono M, Oka S, Okudaira H, et al. [(14)C]Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl Med Biol 2015;42:887-92. [Crossref] [PubMed]
- McParland BJ, Wall A, Johansson S, et al. The clinical safety, biodistribution and internal radiation dosimetry of [18F]fluciclovine in healthy adult volunteers. Eur J Nucl Med Mol Imaging 2013;40:1256-64. [Crossref] [PubMed]
- Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol 2017;197:676-83. [Crossref] [PubMed]
- Suzuki H, Inoue Y, Fujimoto H, et al. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate cancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol 2016;46:152-62. Erratum in: Jpn J Clin Oncol 2017;47:283. [Crossref] [PubMed]
- Sörensen J, Owenius R, Lax M, et al. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging 2013;40:394-402. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. Erratum in: Int J Surg 2010;8:658. [Crossref] [PubMed]
- Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 2016;43:1773-83. [Crossref] [PubMed]
- Kairemo K, Rasulova N, Partanen K, et al. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. Biomed Res Int 2014;2014. [Crossref] [PubMed]
- Odewole OA, Oyenuga OA, Tade F, et al. Reproducibility and reliability of anti-3-[18F]FACBC uptake measurements in background structures and malignant lesions on follow-up PET-CT in prostate carcinoma: an exploratory analysis. Mol Imaging Biol 2015;17:277-83. [Crossref] [PubMed]
- Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology 2011;259:852-61. [Crossref] [PubMed]
- Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 2014;191:1446-53. [Crossref] [PubMed]
- Calais J, Fendler WP, Herrmann K, et al. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J Nucl Med 2018;59:789-94. [Crossref] [PubMed]
- Chaussé G, Abikhzer G, Probst S. Small Lymph Node Metastasis Detected by 68Ga-Prostate-Specific Membrane Antigen But Not 18F-Fluciclovine PET/CT in Low-Prostate-Specific Antigen Biochemical Recurrence of Prostate Cancer. Clin Nucl Med 2018;43:250-1. [PubMed]
- Calais J, Fendler WP, Herrmann K, et al. Reply: Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence: Prospective Trial Is on Its Way. J Nucl Med 2018;59:861. [Crossref] [PubMed]
- Gorin MA, Pienta KJ, Pomper MG, et al. Prostate Cancer Local Recurrence Detected With Both (18)F-Fluciclovine and PSMA-targeted (18)F-DCFPyL PET/CT. Urology 2017;107:e9-e10. [Crossref] [PubMed]
- Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer 2014;12:106-10. [Crossref] [PubMed]
- Nanni C, Zanoni L, Pultrone C, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 2016;43:1601-10. [Crossref] [PubMed]
- Schiavina R, Concetti S, Brunocilla E, et al. First case of 18F-FACBC PET/CT-guided salvage retroperitoneal lymph node dissection for disease relapse after radical prostatectomy for prostate cancer and negative 11C-choline PET/CT: new imaging techniques may expand pioneering approaches. Urol Int 2014;92:242-5. [Crossref] [PubMed]
- Akin-Akintayo O, Tade F, Mittal P, et al. Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol 2018;102:1-8. [Crossref] [PubMed]
- Brunocilla E, Schiavina R, Nanni C, et al. First case of 18F-FACBC PET/CT-guided salvage radiotherapy for local relapse after radical prostatectomy with negative 11C-Choline PET/CT and multiparametric MRI: New imaging techniques may improve patient selection. Arch Ital Urol Androl 2014;86:239-40. [Crossref] [PubMed]
- Schreibmann E, Schuster DM, Rossi PJ, et al. Image Guided Planning for Prostate Carcinomas With Incorporation of Anti-3-[18F]FACBC (Fluciclovine) Positron Emission Tomography: Workflow and Initial Findings From a Randomized Trial. Int J Radiat Oncol Biol Phys 2016;96:206-13. [Crossref] [PubMed]
- Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin Nucl Med 2017;42:e22-8. [Crossref] [PubMed]
- Jani AB, Schreibmann E, Rossi PJ, et al. Impact of (18)F-Fluciclovine PET on Target Volume Definition for Postprostatectomy Salvage Radiotherapy: Initial Findings from a Randomized Trial. J Nucl Med 2017;58:412-8. [Crossref] [PubMed]


