
Single postoperative instillation for non-muscle invasive bladder cancer: are there still any indication?
Introduction
Urinary bladder cancer (BCa) accounts for about 7% of all new cancers in the United States with 81,190 estimated new cases and 17,240 deaths in 2018 (1). Of all patients with BCa, approximately 75% will be diagnosed with a non-muscle-invasive BCa (NMIBC) (2) affected by an average recurrence and progression rates of 60–80% and 10–30%, respectively (3). As a direct consequence of the high recurrence and progression rates, NMIBC accounts for the highest lifetime treatment cost per patient of all cancers (4). Depending on the country, BCa costs from diagnosis-to-death between $89,287 and $202,203 per person and it will likely increase as survival rates increase. Costly surveillance and treatment of BCa can lead to financial toxicity, defined as treatment-related financial distress, with an estimated rate of 24% (5).
Optimization of treatment and surveillance intervals is pivotal to reduce recurrence and progression and to optimize costs. The risk estimation of harboring recurrence or progression depends on multiple factors and several classifications have been proposed to stratify patients, such as EORTC Genito-Urinary Cancer Group scoring system (6) and CUETO risk calculators (7).
Intravesical chemotherapeutical agents after transurethral resection (TURB) have shown to be effective on reducing the risk of recurrence and progression during the follow up (8). Specifically, an early single chemotherapeutical instillation (SI) might play a role for certain patients. On the other hand, the efficacy of this treatment has been questioned and many urologist are reluctant to its use (9,10). For all these reasons, we sought to review and summarize the current evidence regarding the possible impact on recurrence and progression of SI.
Evidence acquisition
A non-systematic Medline/PubMed literature search was performed with different combination of terms as “bladder cancer”, “early instillation”, “single instillation”, “Immediate instillation”, “chemotherapy”, “TURB”, “Epirubicin”, “Mitomycin” and “non-muscle invasive bladder cancer”. Only articles in English language were retained for the review. Time period included articles between 1988 and 2018. Meta-analyses and original articles on randomized controlled trials comparing a single instillation of chemotherapy after TURB and TURB alone or TURB plus placebo or TURB with delayed instillation with endpoints recurrence and progression rate were selected and assessed from authors’ bibliographies.
Overview of the management of NMIBC and risk grouping
Most of newly diagnosed cases of BCa require a conservative management (2). The final decision on subsequent diagnostic/therapeutic process is usually based upon specimen obtained at TURB. To optimize treatment, patients could be stratified in risk classes to predict separately short- and long-term recurrence and progression risk. Patients are stratified in 3 risks group (low, intermediate and high) based on several pathological and clinical features with some differences among available guidelines: EAU (European Association of Urology) (2), National Institute for Health and Care Excellence (NICE), Canadian Urological Association (CUA) (11) and American Association of Urology (AUA) (12) incorporate this strategy into their guidelines whereas National Comprehensive Cancer Network (NCCN) (13) does not. All guidelines recommend SI for certain categories of patients. Figure 1 illustrates a flow chart with summary data regarding the therapeutical management of BCa after TURB.
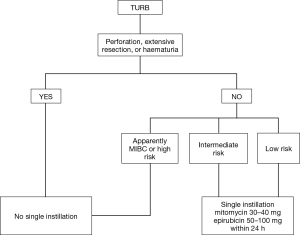
EAU guidelines (2) recommend intravesical single instillation for all patients with low risk tumors [according to EORTC score (6)] after a complete TURB. Instead, for patients with intermediate risk, one-year full dose BCG treatment or instillations of chemotherapy should be provided with the addition of one SI in patients with previous recurrence rate ≤1 per year and expected EORTC recurrence score <5. In the AUA guidelines (12) SI is suggested for patients with suspected or known low- or intermediate-risk BCa whereas CUA (11) and NICE guidelines recommends SI in all patients with a suspected NMIBC. In all guidelines except for NICE it is underlined that SI should not be administered in extended resections or in suspicion of bladder perforation.
The effect of single postoperative instillation after TUR
Table 1 illustrates a selection of randomized control trials testing the impact of SI on recurrence and progression rates. In the last 15 years, several meta-analyses were published testing the effect of SI in BCa patients treated with TURB. Sylvester et al. (30) analyzed in 2004, 1,476 patients affected by TaT1 single and multiple BCa with a median follow up of 3.4 years. Patients treated with SI recorded a decrease of 39% recurrence risk [odds ratio (OR) 0.61, confidence interval (CI): 0.49–0.75, P<0.0001]. The effect was similar between trials using epirubicin, mitomycin C and pirarubicin whereas no benefit was observed using thiotepa. Abern et al. (31) analyzed data from 18 randomized trials with a total of 3,103 patients reporting an absolute reduction of 13% in recurrence for patients who received immediate SI. Perlis et al. (32) in a meta-analysis published in 2013 found a prolonged recurrence free interval for patients treated with SI by 38% (HR: 0.62, 95% CI: 0.50–0.77; P<0.001) (32). The most recent meta-analysis was performed on individual patients’ data (n=2,278) obtained from 11 randomized studies comparing TURB with TURB plus SI. The difference in time of first recurrence between treatments was statistically significant in favor of single instillation, with a reduction of 35% in relative risk of recurrence [hazard ratio (HR): 0.65, 95% CI: 0.58–0.74, P<0.001]. The 5-years recurrence rates were 44.8% (95% CI: 41.6–48.0%) on a single instillation and 58.8% (95% CI: 55.7–61.9%) on TURB (33). The last randomized trial (29) published in the literature compared patients who underwent immediate single instillation (given within <24 h) with Mitomycin C versus delayed instillation (2 weeks after TURB). At 3 years of follow up, there was a recurrence risk of 27% (95% CI: 24–30%) in the immediate group versus 35% (95% CI: 33–39%) for the delayed group with a 34% of relative risk reduction on recurrence (HR: 0.66, 95% CI: 0.56–0.79, P<0.001) (29). To summarize, results of randomized controlled trials and meta-analysis strongly support that SI of chemotherapeutical agent reduces recurrence in NMIBC, with about 35% of relative reduction rates. Theories explaining this effect include the prevention of implantation of floating cells into the bladder urothelium following resection (34-36), and the ablative effect on residual tumor cells at the tumor site and on small unnoticed tumors left behind TURB. It should be emphasized that in the majority of the studies included in this review, patients were strictly selected including only low grade and stage tumors. Few data exist regarding high-risk BCa patients. Bosschieter et al. (29) in a sub-analysis, considered patients based upon their risk group and report a benefit of SI even in the high-risk subgroup (at 3 years of follow up recurrence rates were for SI vs. delayed instillation, 28% vs. 35%, P=0.007).
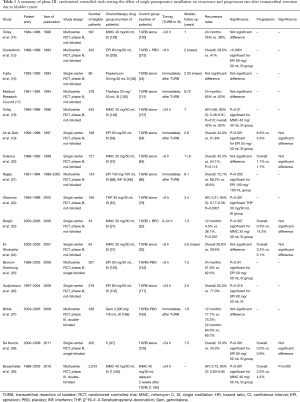
Full table
Some authors analyzed the impact of SI on the bases of tumor characteristics, such as size and numbers of tumors. Berrum-Svennung et al. (25) reported that only patients with tumors <5 mm might benefit from SI in reducing recurrence whereas no differences were found in tumors >5 mm. Gudjónsson et al. (26) reported no recurrence rates benefit for SI in patients with multiple and recurrent tumors; these results were corroborated by a meta-analyses published in 2004 (30). In the last meta-analysis published by Sylvester et al. (33) a subgroup of patients (those with multiple tumors, tumors ≥3 cm, T1 or high recurrent tumors) did not benefit from SI and this indication was included in the EAU and AUA guidelines (2,12,33). Considering these results, patients with single, <3 cm and low-intermediate stage and grade seem most suitable to benefit from SI after TURB with about 35% of relative reduction rates.
Timing of postoperative instillation
The EAU guidelines state that the preferred time window for an immediate instillation is within 2 hour after TURB.
Some discordance exists in the current guidelines regarding the best timing for postoperative instillations. EAU Guidelines state that the preferred time-window for an immediate instillation is within 2 hour after TURB (2). This recommendation is based on the last meta-analysis published where a non-randomized comparison suggests that the instillation should be more effective when given within 2 hours after surgery (33). CUA panel (11) suggest that the optimal timeframe is within 6 and 24 h whereas AUA guidelines proposes to administer the drug within 24 hours after TURB (12). Despite many randomized controlled trials have adopted the policy of giving early instillations immediately or within 6–24 h after TURB, there are only a few randomized studies that have analyzed the impact of timing of SI on BCa recurrence. In addition to Sylvester et al. (33), Hendricksen et al. (37) did not find benefit in recurrence rates when SI was given the day after TURB whereas SI was effective when SI was given on the same day as TURB. On the contrary Gudjónsson et al. (26) found a benefit in recurrence free-survival both in patients in which SI was administered the same day of TURB and in patients in which SI was given the day after TURB (within 24 hours). The same results were observed in a recent sub-analyses obtained from a prospective multicenter randomized trial published in 2018 which evaluated differences between early instillation of mitomycin C administrated within 24 h after TURB on the day of TURB (very early instillation) and administration within 24 h 1 day after TURB (early instillation) (29). More studies required to elucidate the real optimal timing of early instillation.
Drugs in postoperative instillation
Several drugs have been administered in randomized controlled trials testing the effect of SI on recurrence and progression after TURB. Gemcitabine was tested by Böhle et al. (27) without finding any difference in recurrence rates between treatment group and placebo. These results were confirmed in Abern et al. (31) meta-analysis. Studies analyzing thiotepa and peplomycin did not reveal beneficial effects regarding recurrence, in contrast to pirarubicin that was tested by Okamura et al. (22) observing significant improvement of recurrence rates when compared to TURB alone. Mitomycin and epirubicin remain at the moment the mostly widely tested drugs for SI with significant advantages on recurrence rates (14,15,18-21,23-26,28,29). Their efficacy was also confirmed by Perlis et al. (32) and Sylvester et al. (33) meta-analysis. For these reasons, the majority of guidelines suggest the use of one of these two drugs for SI. However, at the time, there is no randomized controlled trial comparing the type of drug on the efficacy of SI in preventing recurrence or progression.
Adverse events after single instillation
None of the analyzed randomized trial showed (Table 1) severe adverse events. The most common side effects included chemical cystitis and skin irritation. For both these adverse event incidences were generally low, but rates also depend on the type of drug used and its dosage. Chemical cystitis occurred with a very low incidence (0.7%) in patients treated with mitomycin by Tolley et al. (14) whereas rates were higher in a publication of De Nunzio et al. (28) (about 9%) and Bosschieter et al.(29) (about 5%). About 11.7% of patients treated with epirubicin 80 mg by Oosterlinck et al. (15) developed chemical cystitis. Despite the relatively high dose of epirubicin (100 mg) administrated by Rajala et al. (21) no considerable side effects were found. Solsona et al. (20) reported that 3.5% of patients treated with mitomycin developed chemical cystitis and allergic skin reaction. In Bosschieter et al. (29) analysis, the most reported adverse event in SI with mitomycin was exanthema (5.4%)
No patient treated with SI of Peplomycin 80 mg developed side effect (16) whereas about 2.5% of patients treated with SI of Thiotepa 30 mg developed chemical cystitis (17). Böhle et al. (27) found that adverse events possibly related to instillation with gemcitabine treatment were rare (6.6% of patients treated with gemcitabine, all non-serious).
Although all of these trials showed a beneficial safety profile of intravesical SI a few case reports of severe complication are reported in literature. Nieuwenhuijzen et al. (38) reported a case of extravasation of mitomycin after instillation, resulting in severe continuous pelvic pain that required surgical debridement. Oddens et al. (39) reported three cases of severe complications: extravasation (which has been treated with prolonged catheterization, antibiotics and analgesics), abdominal pain (associated with a CT scan positive for an infiltrate mass between abdomen and abdominal wall without sign of collection treated conservatively) and a paralytic ileus. For all these reasons, it is strongly recommended to omit SI in any case of overt or suspected bladder perforation or bleeding requiring bladder irrigation.
Conclusions
Randomized controlled trials and meta-analyses strongly support the use of SI after TURB in preventing recurrence in low and intermediate risk patients. This effect is mainly evident in small (<3 cm) and solitary tumors with a recurrence reduction of 35%. No randomized trials compared the effect of different type of drugs on recurrence, although epirubicin or mitomycin C seems associated with an improved recurrence effect. It is advisable to administer SI within 24 h from TURB, even if the optimal timeframe has not yet been established. Although is a safe procedure, SI should not be administered in patients who underwent extended resections or if bladder perforation is suspected since extravasation could cause potential deadly complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol 2000;163:60-1; discussion 61-2. [Crossref] [PubMed]
- Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol 2014;24:487-91. [Crossref] [PubMed]
- Casilla-Lennon MM, Choi SK, Deal AM, et al. Financial Toxicity among Patients with Bladder Cancer: Reasons for Delay in Care and Effect on Quality of Life. J Urol 2018;199:1166-73. [Crossref] [PubMed]
- Maffezzini M. Re: Richard J. Sylvester, Adrian P.M. van der Meijden, Willem Oosterlinck, J. Alfred Witjes, Christian Bouffioux, Louis Denis and Donald W.W. Newling. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-77. Eur Urol 2006;50:623-4; author reply 624-5. [Crossref] [PubMed]
- Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol 2009;182:2195-203. [Crossref] [PubMed]
- Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res 2001;21:765-9. [PubMed]
- Palou-Redorta J, Rouprêt M, Gallagher JR, et al. The use of immediate postoperative instillations of intravesical chemotherapy after TURBT of NMIBC among European countries. World J Urol 2014;32:525-30. [Crossref] [PubMed]
- Cookson MS, Chang SS, Oefelein MG, et al. National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol 2012;187:1571-6. [Crossref] [PubMed]
- Kassouf W, Traboulsi SL, Kulkarni GS, et al. CUA guidelines on the management of non-muscle invasive bladder cancer. Can Urol Assoc J 2015;9:E690-704. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Spiess PE, Agarwal N, Bangs R, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1240-67. [Crossref] [PubMed]
- Tolley DA, Hargreave TB, Smith PH, et al. Effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: interim report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). Br Med J (Clin Res Ed) 1988;296:1759-61. [Crossref] [PubMed]
- Oosterlinck W, Kurth KH, Schröder F, et al. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol 1993;149:749-52. [Crossref] [PubMed]
- Fujita K. Intravesical antitumor therapy immediately after transurethral resection of bladder cancer. Int J Urol 1994;1:341-4. [Crossref] [PubMed]
- The effect of intravesical thiotepa on tumour recurrence after endoscopic treatment of newly diagnosed superficial bladder cancer. A further report with long-term follow-up of a Medical Research Council randomized trial. Medical Research Council Working Party on Urological Cancer, Subgroup on Superficial Bladder Cancer. Br J Urol 1994;73:632-8. [Crossref] [PubMed]
- Tolley DA, Parmar MK, Grigor KM, et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol 1996;155:1233-8. [Crossref] [PubMed]
- Ali-el-Dein B, Nabeeh A, el-Baz M, et al. Single-dose versus multiple instillations of epirubicin as prophylaxis for recurrence after transurethral resection of pTa and pT1 transitional-cell bladder tumours: a prospective, randomized controlled study. Br J Urol 1997;79:731-5. [Crossref] [PubMed]
- Solsona E, Iborra I, Ricós JV, et al. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol 1999;161:1120-3. [Crossref] [PubMed]
- Rajala P, Kaasinen E, Raitanen M, et al. Perioperative single dose instillation of epirubicin or interferon-alpha after transurethral resection for the prophylaxis of primary superficial bladder cancer recurrence: a prospective randomized multicenter study--FinnBladder III long-term results. J Urol 2002;168:981-5. [Crossref] [PubMed]
- Okamura K, Ono Y, Kinukawa T, et al. Randomized study of single early instillation of (2"R)-4'-O-tetrahydropyranyl-doxorubicin for a single superficial bladder carcinoma. Cancer 2002;94:2363-8. [Crossref] [PubMed]
- Barghi MR, Rahmani MR, Hosseini Moghaddam SM, et al. Immediate intravesical instillation of mitomycin C after transurethral resection of bladder tumor in patients with low-risk superficial transitional cell carcinoma of bladder. Urol J 2006;3:220-4. [PubMed]
- El-Ghobashy S, El-Leithy TR, Roshdy MM, et al. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term follow-up. J Egypt Natl Canc Inst 2007;19:121-6. [PubMed]
- Berrum-Svennung I, Granfors T, Jahnson S, et al. A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. J Urol 2008;179:101-5; discussion 105-6. [Crossref] [PubMed]
- Gudjónsson S, Adell L, Merdasa F, et al. Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol 2009;55:773-80. [Crossref] [PubMed]
- Böhle A, Leyh H, Frei C, et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, double-blind, placebo-controlled phase III multicentre study. Eur Urol 2009;56:495-503. [Crossref] [PubMed]
- De Nunzio C, Carbone A, Albisinni S, et al. Long-term experience with early single mitomycin C instillations in patients with low-risk non-muscle-invasive bladder cancer: prospective, single-centre randomised trial. World J Urol 2011;29:517-21. [Crossref] [PubMed]
- Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, et al. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur Urol 2018;73:226-32. [Crossref] [PubMed]
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171:2186-90, quiz 2435. [Crossref] [PubMed]
- Abern MR, Owusu RA, Anderson MR, et al. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. J Natl Compr Canc Netw 2013;11:477-84. [Crossref] [PubMed]
- Perlis N, Zlotta AR, Beyene J, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol 2013;64:421-30. [Crossref] [PubMed]
- Sylvester RJ, Oosterlinck W, Holmang S, et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur Urol 2016;69:231-44. [Crossref] [PubMed]
- Soloway MS, Nissenkorn I, McCallum L. Urothelial susceptibility to tumor cell implantation: comparison of cauterization with N-methyl-N-nitrosourea. Urology 1983;21:159-61. [Crossref] [PubMed]
- Soloway MS, Masters S. Urothelial susceptibility to tumor cell implantation: influence of cauterization. Cancer 1980;46:1158-63. [Crossref] [PubMed]
- Weldon TE, Soloway MS. Susceptibility of urothelium to neoplastic cellular implantation. Urology 1975;5:824-7. [Crossref] [PubMed]
- Hendricksen K, Witjes WP, Idema JG, et al. Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. Eur Urol 2008;53:984-91. [Crossref] [PubMed]
- Nieuwenhuijzen JA, Bex A, Horenblas S. Unusual complication after immediate postoperative intravesical mitomycin C instillation. Eur Urol 2003;43:711-2. [Crossref] [PubMed]
- Oddens JR, van der Meijden AP, Sylvester R. One immediate postoperative instillation of chemotherapy in low risk Ta, T1 bladder cancer patients. Is it always safe? Eur Urol 2004;46:336-8. [Crossref] [PubMed]


