A pictorial review of bladder cancer nodal metastases
Introduction
Bladder cancer is the 9th most common cause of cancer worldwide and the second most common genitourinary malignancy (behind prostate cancer) (1,2). Most patients with bladder cancer present with hematuria. However, only 10% of patients with gross hematuria and 2% of patients with microscopic hematuria are caused by bladder cancer (3).
Lymph node involvement in bladder cancer occurs in approximately 30% of cases invading the bladder wall (stage T2) and 60% of cases in which the cancer extends into the perivesical tissue (stage T3 or greater) (4,5). Early and accurate identification of metastatic lymph nodes is important in ensuring that the appropriate patients are triaged to surgery. It may also alter the lymphadenectomy template in those patients who do undergo surgery. This can also expedite definite care in cases where the local staging is equivocal, particularly if nodal disease can be definitively identified (4,6,7).
Five-year disease-free survival rates following treatment of bladder cancer can be as great as 89%, depending on the extent of local involvement at presentation (2,6). However, the presence of nodal involvement is associated with a pronounced decrease in 5-year disease-free survival, to approximately 35% (2,6). With this in mind, the role of imaging in assisting in accurate detection and staging of nodal disease in bladder cancer is an important driver in ensuring timely and appropriate patient care and in establishing accurate prognoses (4,8).
The purpose of this review is to provide a pictorial and educational overview of the staging of and imaging appearance of metastatic lymph nodes in bladder cancer and to provide a summary of the diagnostic accuracy of common imaging modalities in detecting metastatic lymph nodes in bladder cancer patients.
Methods
This was an Institutional Review Board-approved Health Insurance Portability and Accountability Act compliant retrospective educational pictorial review. The requirement for informed consent was waived. Images obtained for this review were derived from authors personal teaching files and a retrospective review of lymph node pathology from the electronic medical record at a large academic quaternary care center between 1/1/2010 and 12/31/2017 (9).
Review
Lymphatic drainage of the bladder
The lymphatic drainage from the bladder involves many regions within and beyond the pelvis (10). The specific route of lymphatic spread from the bladder is complex and impacted by the primary location of the tumor (10,11). Roth et al. performed a prospective study of 60 patients to assess the primary lymphatic landing site from the bladder and showed that while the major lymphatic landing sites are regional lymph nodes, a small majority of cases showed drainage initially to more distant lymph nodes, such as the common iliac (15%) or paraortic (4%) regions (10). Given this pattern, it is not surprising that the most commonly effected sites of nodal involvement are the obturator and internal iliac regions (10,12,13).
Lymph node staging of bladder cancer
Staging of nodal metastases from bladder cancer is based on the current TNM guidelines from the American Joint Committee on Cancer, eight edition staging manual (AJCC 8th edition) (8,14,15). Definitive lymph node staging is based on post-operative histology and should only be suggested on imaging studies when lymph node involvement is obvious. Nodal staging, which is provided in Figure 1, is also summarized as follows:
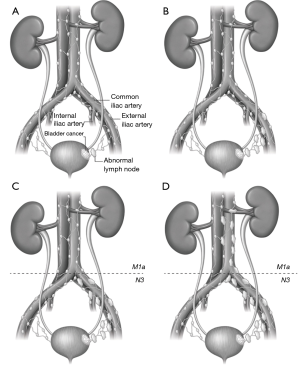
- N0 disease is defined as no lymph node involvement;
- N1 disease is defined as bladder cancer involvement of one regional pelvic lymph node. Regional lymph nodes include: inguinal, hypogastric (internal iliac chain), obturator, external iliac chain, perivesical, and presacral lymph nodes, all of which are located below the level of the common iliac arteries (Figure 1) (8,14-16);
- N2 disease is defined as involvement of more than one regional lymph node. It should be noted that in previous staging classification systems, differences in involved lymph node size were also used to differentiate N1 from N2 disease; however, lymph node size is no longer considered part of the staging system (Figure 1) (8,15).
- N3 disease is defined as a positive lymph node along either or both of the common iliac chains (Figure 1) (8,14,15);
- Importantly, involved lymph nodes, above the level of the aortic bifurcation, are now considered to be distant metastases and classified as M1a disease. Metastases to other organs are now classified as M1b disease) (Figure 1) (8,14,15).
The location and number of involved lymph nodes can be helpful in predicting the prognosis for patients with advanced disease (8).
Lymph node evaluation on CT
The most commonly used first line imaging study for evaluation of patients with suspected or known newly diagnosed bladder cancer is CT of the abdomen and pelvis, typically in the form of a CT urogram (5,17,18). The CT urogram, performed without and with intravenous contrast material, is considered the appropriate first line test for the detection of suspected bladder cancer in patients with either microscopic or gross hematuria and is also considered an appropriate first step in staging for those patients in whom invasive bladder cancer (stage T2 or greater) has been diagnosed at cystoscopy (17-19).
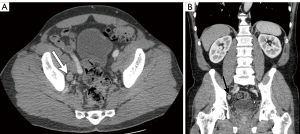
While the use of lymph node size alone (measured in maximal short axis diameter) had historically been a marker of nodal involvement and was used as a measure of nodal staging in prior iterations of the AJCC TNM criteria (5), size criteria alone, as a marker of disease involvement is imprecise due to the wide range of normal lymph node sizes and the potential for metastatic bladder cancer occurring within non-enlarged lymph nodes (Figures 2,3) (12). Acknowledging these limitations, recommended size thresholds that have been utilized are ≥8 mm in short axis for suspected abnormal pelvic lymph nodes and ≥10 mm in short axis for abdominal lymph nodes (Figure 4) (12).
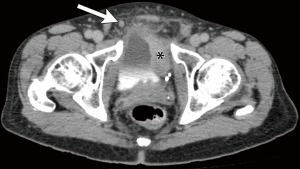
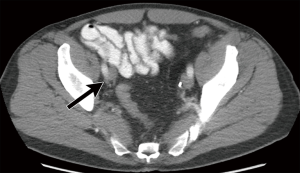
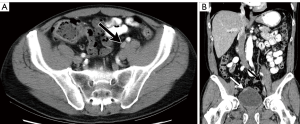
For the previously provided reasons, some investigators have found that identification of suspected metastatic lymph nodes on CT is most accurate when lymph node features, in addition to size, are assessed. This includes lymph node shape and internal architecture/attenuation (12). Normal lymph nodes generally have a reniform shape, as they frequently contain a central fatty hilum. The loss of this normal appearance and a more rounded (Figure 2) or irregular configuration should be concerning for involvement with metastatic tumor (Figure 5) (12,20). Another alteration of internal nodal architecture, is central low attenuation due to necrosis, which should be considered suspicious for metastatic disease when a primary pelvic neoplasm is present (21).
There has been limited study of the diagnostic accuracy of CT in nodal staging of bladder cancer over the past 10 years (6,22-25), with the few recent publications having found that even with current techniques, the sensitivity of CT in detecting metastatic bladder cancer in lymph nodes is highly variable, but generally limited, ranging from 30% to 75%. The limited sensitivity is likely primarily due to the presence of metastases within normal sized lymph nodes (high proportion of false negatives). There has been a wide range in reported specificities, from 56% to 100% (6,22-25). Overall diagnostic accuracy in identifying N1, N2, or N3 bladder cancer has recently been reported at between 61% and 97% (4). Overall, CT can be viewed as more specific than sensitive for the detection of nodal involvement.
Lymph node evaluation on multiparametric magnetic resonance imaging (MRI)
MRI of the of the abdomen and pelvis is an alternative, but less commonly utilized, modality for evaluating lymph nodes in patients with suspected or known bladder cancer (18). When consideration is made as to whether or not to obtain an MRI, the potential benefits of multiparametric assessment of nodal characteristics, should be weighed against a number of potential contraindications, including the patient’s ability to lie still for a prolonged period of time, claustrophobia, the presence of regional orthopedic hardware, and potential MR incompatibility of any implantable medical devices that the patient may have (7).
The role of MRI in assessment of nodal involvement is an area of active investigation (26). Similar to CT, metastatic lymph nodes are conventionally identified when short axis diameter lymph node exceeds 8–10 mm (12); however, assessment of nodal size by MRI suffers from the same limitations as CT, in that normal sized lymph nodes may still harbor metastatic disease (4,13,20).
Compared with CT, the use of multiple pulse sequences in MRI allows for evaluation of a large number additional lymph node features independent of size, potentially aiding in the identification or exclusion of metastatic disease (7). Some of the many other multiparametric MRI lymph node features that can be evaluated include: lymph node shape, T1 and T2 signal intensity, diffusion characteristics, and the temporal nature of gadolinium-based contrast material enhancement. On MRI, normal lymph nodes often demonstrate a reniform configuration with a preserved central T1-hyperintense fatty hilum (Figure 6). Metastatic lymph nodes are more likely to demonstrate heterogeneous and/or increased peripheral enhancement (Figures 7,8) and heterogeneous central T1-hypointensity and T2-hyperintensity, corresponding to necrosis (Figure 9) (21). To date, no routinely acquired MRI sequence (T1-weighted, T2-weighted, diffusion weighted or gadolinium-enhanced) has been shown to be superior to the others in terms of increasing sensitivity or specificity in the detection of metastatic lymph nodes (7,13,26).
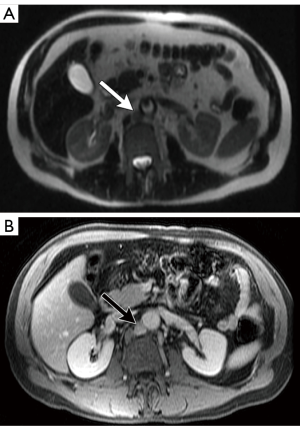
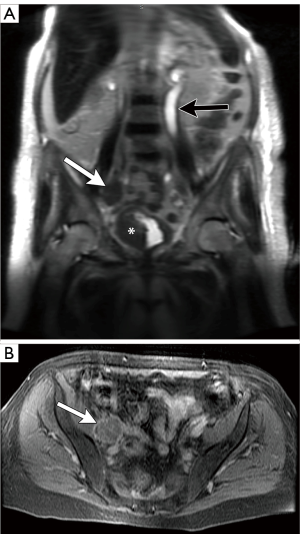

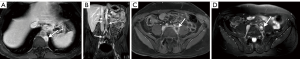
Despite the potential advantages of MRI over CT in assessing lymph nodes in bladder cancer patients, studies evaluating the ability of MRI to detect metastatic lymph nodes have not shown significant diagnostic benefits, especially with respect to sensitivity. A recent meta-analysis by Woo et al. showed a pooled sensitivity of MRI for identifying metastatic lymph nodes from bladder cancer to be 56%, although the specificity was 94% (26). In another meta-analysis, Salminen et al. showed a sensitivity range of 40.7–86% and a specificity of 31–92% (with 5/6 included studies having a specificity >80%) (7).
The use of intravenous lymphotropic ultrasmall superparamagnetic particles of iron oxide (USPIO) in further characterization of metastatic lymph nodes has been suggested as a possible alternative technique for improving accuracy in detection of metastatic lymph nodes (12,27,28). USPIO functions by being taken up in benign lymph nodes, which appear diffusely hypointense on T2-weighted and gradient echo sequences (12,27-29). In contrast, metastatic lymph nodes remain relatively hyperintense on these sequences. The use of USPIO has been shown in a recent meta-analysis by Woo et al. to improve pooled sensitivity to 86% (from 56%), while pooled specificity is relatively unchanged at 93%, with similar trends having been shown in other studies (7,26,29-31). However, USPIO is not approved for routine clinical use within the United States at this time and may require logistic hurdles due to the potential need for multiple separate episodes of scanning (7).
Lymph node evaluation on positron emission tomography-CT (PET/CT)
PET/CT is a potentially helpful modality for improving sensitivity in the detection of metastatic lymph nodes in bladder cancer patients. The ACR appropriateness criteria lists PET/CT in the category of “may be appropriate” for bladder cancer staging (18). Due to physiologic excretion of radiotracer into the urinary tract, however, 18-Fluoro-deoxyglucose (FDG) PET/CT is of limited utility in local staging (4,7,18,22).
Metastatic lymph nodes have increased metabolic activity and, therefore, demonstrate increased radiotracer uptake on PET/CT (Figures 10,11) (4). Unfortunately, due to limitations in spatial resolution of PET, PET/CT has decreased accuracy in the detection of disease in non-enlarged but metastatic lymph nodes (<5 mm) (12). Furthermore, increased radiotracer uptake can be seen in lymph nodes that do not harbor metastatic disease (i.e., reactive or inflammatory lymph nodes). Thus, there are many opportunities for both false negative and false positive PET/CT results in bladder cancer patients.

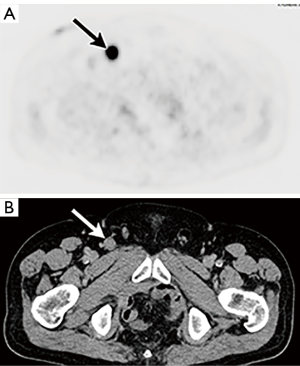
Assessment of the accuracy of PET/CT in the detection of nodal disease in bladder cancer has been an area of growing interest and investigation. As with CT and MRI, many studies have shown that PET/CT has limited sensitivity, but much better specificity in detecting and excluding lymph node metastases. A meta-analysis performed by Soubra et al. showed FDG PET demonstrated sensitivities in detecting bladder cancer lymph node metastases of 33–78% and specificities of 86.7–100% (32). A prospective study and meta-analysis performed by Jeong et al. showed pooled sensitivities of 47% to 70% and specificities of 87% to 100% (33). A study of 233 patients by Goodfellow showed PET/CT had an improved sensitivity compared to conventional CT (69% vs. 45%), but resulted in a slight decrease in specificity (95% vs. 98%) (34).
C11-Choline PET has been studied as a potential alternative tracer to be used in bladder cancer imaging due its limited urinary excretion (35). Kim et al. performed a meta-analysis of 10 studies on the diagnostic accuracy of C11-choline PET in the detection of nodal metastases from bladder cancer and found a pooled sensitivity of only 66% and a pooled specificity of 89% (35).
Conclusions
Understanding changes in nodal staging guidelines is important for radiologists and urologists engaged in imaging review, in order to understand the staging implications based on the location and number of abnormal lymph nodes. Lymph node metastases from bladder cancer are also an important marker of disease prognosis. While, radiologists historically have relied on size, a combination of lymph node size, shape and internal architecture is most effective in identifying potentially metastatic lymph nodes. Conventional CT remains the mainstay of imaging for patients with known or suspected bladder cancer, likely due to its speed and easy availability, and can be viewed as more specific than sensitive for the detection of lymph node metastases. MRI and PET/CT have demonstrated better sensitivity than CT; however, the sensitivity of these studies in detecting lymph node metastases is still limited. Additional research is still necessary to demonstrate the potential impact these imaging modalities may have on patient management. Further investigation is needed to improve diagnostic accuracy and to balance any potential improvements with imaging costs and patient preferences.
Acknowledgements
The authors would like to acknowledge and thank Ms. Danielle Dobbs of the University of Michigan Media Division for efforts in figure creation and optimization for this manuscript. The authors would also like to acknowledge and thank Mr. Mark MacEachern of University of Michigan Taubman Health Sciences Library for his efforts in assisting with literature review for this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Medical School Institutional Review Board, University of Michigan (HUM00145635) and written informed consent was waived.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- NCI SEER Database. Cancer Stat Facts: Bladder Cancer. National Cancer Institute, 2018. Available online: https://seer.cancer.gov/statfacts/html/urinb.html
- Elias K, Svatek RS, Gupta S, et al. High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer 2010;116:2954-9. [Crossref] [PubMed]
- Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: part 1, lower urinary tract. AJR Am J Roentgenol 2009;192:1481-7. [Crossref] [PubMed]
- MacVicar AD. Bladder cancer staging. BJU Int 2000;86 Suppl 1:111-22. [Crossref] [PubMed]
- Horn T, Zahel T, Adt N, et al. Evaluation of Computed Tomography for Lymph Node Staging in Bladder Cancer Prior to Radical Cystectomy. Urol Int 2016;96:51-6. [Crossref] [PubMed]
- Salminen AP, Jambor I, Syvanen KT, et al. Update on novel imaging techniques for the detection of lymph node metastases in bladder cancer. Minerva Urol Nefrol 2016;68:138-49. [PubMed]
- Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol 2018;73:560-9.
- Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: A report of University of Michigan's nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform 2015;55:290-300. [Crossref] [PubMed]
- Roth B, Wissmeyer MP, Zehnder P, et al. A new multimodality technique accurately maps the primary lymphatic landing sites of the bladder. Eur Urol 2010;57:205-11. [Crossref] [PubMed]
- Paño B, Sebastià C, Buñesch L, et al. Pathways of Lymphatic Spread in Male Urogenital Pelvic Malignancies. RadioGraphics 2011;31:135-60. [Crossref] [PubMed]
- McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 2010;254:31-46. [Crossref] [PubMed]
- Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: role of MR imaging. Radiographics 2012;32:371-87. [Crossref] [PubMed]
- Robins DJ, Small AC, Amin MB, et al. MP86-17 The 2017 American Joint Committee on Cancer Eighth Edition Cancer Staging Manual: Changes in Staging Guidelines for Cancers of the Kidney, Renal Pelvis and Ureter, Bladder and Urethra. J Urol 2017;197:e1163.
- AJCC. AJCC Cancer Staging Manual. 8 ed. Springer International Publishing, 2017.
- Paner GP, editor. Updates in new AJCC TNM Staging of Bladder Cancer: Issues Pertaining to Application in Routine Surgical Pathology. San Antonio, TX: United States and Candian Academy of Pathology, 106th Annual Meeting, 2017.
- ACR Appropriateness Criteria: Hematuria. American College of Radiology, 2014. Available online: https://acsearch.acr.org/docs/69490/Narrative/
- ACR Appropriateness Criteria: Pretreatment Staging of Muscle-Invasive Bladder Cancer. American College of Radiology, 2017. Available online: https://acsearch.acr.org/docs/69370/Narrative/
- Rajesh A, Sokhi H, Fung R, et al. Role of whole-body staging computed tomographic scans for detecting distant metastases in patients with bladder cancer. J Comput Assist Tomogr 2011;35:402-5. [Crossref] [PubMed]
- Jager GJ, Barentsz JO, Oosterhof GO, et al. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am J Roentgenol 1996;167:1503-7. [Crossref] [PubMed]
- Yang WT, Lam WW, Yu MY, et al. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol 2000;175:759-66. [Crossref] [PubMed]
- Maurer T, Souvatzoglou M, Kubler H, et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol 2012;61:1031-8. [Crossref] [PubMed]
- Baltaci S, Resorlu B, Yagci C, et al. Computerized tomography for detecting perivesical infiltration and lymph node metastasis in invasive bladder carcinoma. Urol Int 2008;81:399-402. [Crossref] [PubMed]
- Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol 2012;30:827-31. [Crossref] [PubMed]
- Ficarra V, Dalpiaz O, Alrabi N, et al. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int 2005;95:786-90. [Crossref] [PubMed]
- Woo S, Suh CH, Kim SY, et al. The Diagnostic Performance of MRI for Detection of Lymph Node Metastasis in Bladder and Prostate Cancer: An Updated Systematic Review and Diagnostic Meta-Analysis. AJR Am J Roentgenol 2018;210. [Crossref] [PubMed]
- Weissleder R, Elizondo G, Wittenberg J, et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 1990;175:489-93. [Crossref] [PubMed]
- Harisinghani MG, Saini S, Weissleder R, et al. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: radiographic-pathologic correlation. AJR Am J Roentgenol 1999;172:1347-51. [Crossref] [PubMed]
- de Haas RJ, Steyvers MJ, Futterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol 2014;202:1187-95. [Crossref] [PubMed]
- Birkhauser FD, Studer UE, Froehlich JM, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol 2013;64:953-60. [Crossref] [PubMed]
- Brown AM, Sankineni S, Bernardo M, et al. Ferumoxytol enhanced MRI for lymph node staging in prostate cancer. J Clin Oncol 2015;33:208. [Crossref]
- Soubra A, Hayward D, Dahm P, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol 2016;34:1229-37. [Crossref] [PubMed]
- Jeong IG, Hong S, You D, et al. FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol 2015;22:3150-6. [Crossref] [PubMed]
- Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int 2014;114:389-95. [PubMed]
- Kim SJ, Koo PJ, Pak K, et al. Diagnostic accuracy of C-11 choline and C-11 acetate for lymph node staging in patients with bladder cancer: a systematic review and meta-analysis. World J Urol 2018;36:331-40. [Crossref] [PubMed]


