Evaluation of lymph node status in patients with urothelial carcinoma—still in search of the perfect imaging modality: a systematic review
Introduction
Urothelial carcinoma, or transitional cell carcinoma (TCC), comprises a group of heterogeneous malignant diseases typically involving the urinary system. Bladder cancer (BC), being the most common urinary malignancy worldwide, with the urothelial type accounting for 90 percent of cases diagnosed in the United States and Europe, is the ninth leading cause of cancer death in the United States (1,2). With the exception of non-muscle invasive bladder cancer (NMIBC), relatively indolent but still capable of progressing into muscle invasive and metastatic disease (3-5), urothelial carcinomas are known for its rapid metastatic ability (6,7). While the risk of metastatic lymph node involvement (LNI) reaches 10–30% in patients with organ-confined muscle-invasive bladder cancer (MIBC), it increases to 50% in cases when the tumor extends into perivesical fatty tissue (8). It is estimated that 10–15% of eventually metastatic BC patients are metastatic at the time of diagnosis (9).
While cure can be achieved in as much as 75–80% of patients with organ-confined disease, this number drops to about 60% in T3, node-negative disease, and is estimated to be as low as 30% with positive lymph node status (10-14). In patients with LNI the 5-year recurrence-free survival rates are 35% regardless of the T stage (12,15). Due to short survival expectancies metastatic and node-positive patients are generally not suitable for radical treatment (6,7) and have historically been managed as with metastatic disease and included into chemotherapy clinical trials (16). Interestingly, recent studies indicate that lymph node involvement may not inevitably impair the oncologic outcome, and several treatment modalities, including radical cystectomy with or without neoadjuvant chemotherapy, are being used with promising results for clinically node-positive disease (17-19).
Given the fact that LNI has a significant impact on the prognosis and hence alters treatment selection (20,21), accurate staging of the disease with imaging studies is vital in terms of establishing the right management plan. Several different imaging modalities, including computed tomography urography (CTU), magnetic resonance (MR), or positron emission tomography (PET), are being used for the purpose of determining disease stage with variable results. Our objective was to systematically review the up to date literature concerning the role of particular imaging modalities in evaluating lymph node status in urothelial cancer patients.
Materials and methods
The authors of this systematic review adhered to a predefined protocol developed according to PRISMA guidelines. A systematic search of literature using electronic databases was performed in order to review current evidence-based data relating to the topic of this article.
We combined searches from the Medline and Scopus electronic databases. A detailed search query developed for use in PubMed (Medline) was as follows: (bladder cancer[MeSH Terms] OR transitional cell carcinoma[MeSH Terms]) AND “english”[Language] AND (“2003/01/01”[Date, Publication]: “2018/05/30”[Date, Publication]) AND (MRI[Title/Abstract] OR MR[Title/Abstract] OR magnetic resonance[Title/Abstract] OR CT[Title/Abstract] OR computed tomography[Title/Abstract] OR PET-CT[Title/Abstract] OR PET[Title/Abstract] OR positron[Title/Abstract] OR imaging[Title/Abstract] OR uspio[Title/Abstract]). For the purpose or Scopus search we developed a query as follows: TITLE-ABS-KEY(“bladder” OR “transitional” OR “urothelial”) AND TITLE-ABS-KEY(“cancer” OR “carcinoma”) AND TITLE-ABS-KEY(MR* OR “imaging” OR “resonance” OR “CT” OR “tomography” OR “PET” OR “positron” OR “USPIO”), and the search results were restricted to English language papers published between 2003/01/01 and 2018/05/30.
Articles were considered for this review if they: (I) were published in a peer-reviewed English language journal; (II) were either meta-analyses, reviews, clinical trials, cohort studies, case-control studies or cross-sectional studies; (IIIa) reported either sensitivity or specificity of an imaging modality in detecting urothelial carcinoma metastases or (IIIb) reported data on imaging-related understaging or overstaging of urothelial cancer disease. The eligibility of all full-text studies listed in the search results was independently assessed by two reviewers.
Each article meeting the eligibility criteria underwent a quality assessment by the co-authors, in order to exclude studies of relatively small statistical power. Eventually, data from the studies included into this review was abstracted and analyzed.
Computed tomography (CT) (Table 1)

Full table
Multiphase contrast-enhanced CT with urinary excretory phase, or computed tomography urography (CTU), is the imaging modality of choice for the purpose of urinary tract cancer staging (6,7). The recognition of CT as the gold standard clinical staging method is reflected by CT being most commonly compared to in studies that evaluate the utility of other imaging modalities, as revealed in this review.
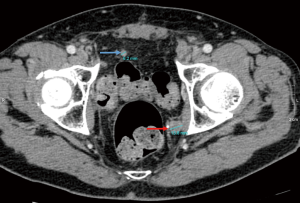
The assessment of the status of a potentially metastatic lymph node is based primarily on the measurement of its diameter, making this approach susceptible to understaging in case of small-size metastatic foci, or overstaging in case of marked nodal inflammatory response. The dimension universally used for measuring the size of a lymph node is its short axis diameter (Figure 1). In one study, Hitier-Berthault et al. (29) demonstrated a remarkably low sensitivity (9.1%) when using the long axis for this purpose. The sensitivity and specificity of CT regarding lymph node status evaluation are greatly dependent on the diameter cut-off values adopted, which may be additionally complicated by the fact that optimal short axis of a lymph node is influenced by its shape (34). The universally recognized threshold value of a lymph node short-axis diameter indicative of metastatic involvement is 8 mm for urothelial carcinoma (6,34). However, both 8 and 10 mm cut-off values were adopted in the studies included in this review, as shown in Table 1. Moreover, Li et al., who retrospectively analyzed data collected from 191 bladder cancer patients, suggested a threshold value of 6.8 mm, as this was associated with the area under receiver operating characteristic (ROC) curve of 0.815, with the increased sensitivity (83.0%) being accompanied by relatively low specificity (64.3%). Apart from the short axis size, the shape of a lymph node may be revealing of its status, with a round shape indicative of metastasis and an oval shape characteristic of an inflammatory response. Hence, the long-to-short axis ratio has been proposed as a measurable indicator of LNI (22). Yet, this parameter has not been shown to improve the diagnostic performance compared to short axis diameter alone (22).
A short-axis diameter cut-off value of 8 mm, while decreasing the false-positive rate, makes it virtually impossible to detect smaller metastases, which may be of dramatic significance at the early stage of metastatic spread. This is reflected by non-satisfactory, low sensitivity values of CT used for the purpose of LN status evaluation in BC patients, which, in studies included into this review, ranged from 14% in a study by Brunocilla (28), to 46% (23,27), with the majority of the values falling between 30% and 40%, as presented in Table 1. On the other hand, CT is characterized by relatively high specificity in regard to the nodal staging of BC, which, according to prospective studies included in this review, demonstrated values between 89% and 100% (23-27,29-33). Among other studies, summarized in Table 1, a retrospective analysis by Horn et al. (24), based on data collected from 231 patients, reported sensitivity values of 30.2% on patient-based level and 52.6% on a field basis, and specificity values of 98.0% and 93.6%, respectively.
The clinical significance of sensitivity and specificity of an imaging modality in regard to LN status evaluation is reflected in the under and overstaging rates. Tritschler et al. (30)., demonstrated in a retrospective study on 276 patients diagnosed with bladder cancer and treated with radical cystectomy with pelvic lymph node dissection, that a preoperative CT scan was associated with LN evaluation-related under and overstaging rates of 29.4% and 8.3%, respectively. Similarly, according to the results of a prospective study by Jeong et al. (25), preoperative LN status assessment using a CT scan led to under and overstaging in 21.3% and 6.6% of cases, respectively.
Magnetic resonance imaging (MRI) (Tables 2,3)
Full table

Full table
Although magnetic resonance (MR) appears to be an excellent imaging modality in terms of local staging of bladder cancer, mainly due to its high soft-tissue contrast resolution, its role in the process of nodal staging of urothelial carcinoma is not definitely established (45-51). While the application of diffusion-weighted (DW) MR imaging (DW-MRI) for the purpose of detecting LN metastases has been shown to be useful in several other malignancies (52-56), the number of studies demonstrating similar clinical significance of MR in urothelial carcinoma patients is limited.
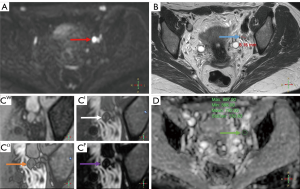
The main imaging-related advantage of MR over CT in terms of LN status evaluation is that MR sprovides an opportunity to assess several features other than the size or shape of a lymph node, namely, the presence of fatty hilum (as loss of fatty hilum is characteristic of metastatic involvement) and diffusion parameters (quantified with the apparent diffusion coefficient, or ADC) (Figure 2), as well as it enables true dynamic contrast enhanced (DCE) imaging in multiple phases without using ionized radiation. As shown in a study by Mir et al. (57), a fusion of high b-value (750 s/mm2) DW-MRI with conventional T2-weighted images could improve lymph node identification. In the study by Li et al. (22) the presence of fatty hilum was indicative of non-metastatic character of a lymph node in bladder cancer patients. Papalia et al. (40) have stated that DW imaging sequences could increase the sensitivity of MR in detecting lymph node metastases to 76%, with the optimal ADC cut-off value of 0.86×10−3 mm3/s. The role of DCE-MR with gadolinium used as the contrast agent has been studied by Daneshmand et al. (39), who prospectively analyzed data from 122 bladder cancer patients, reported the sensitivity, specificity, and accuracy values of preoperative gadolinium DCE-MR imaging for the evaluation of nodal status of 40.7%, 91.5% and 80.3%, respectively.
A distinct method of imaging using MR is using ultra-small superparamagnetic iron oxide (USPIO) as a contrast agent. Following an injection of ferumoxtran-10 solution, a significant drop of signal intensity on T1- and T2-weighted images is observed in non-metastatic lymph nodes, as the contrast is absorbed by local macrophages. On the other hand, the presence of bright signal is indicative of a metastatic tumor growing in the lymph node, as the macrophages have been replaced by metastatic cells and therefore ferumoxtran-10 particles are not cleared (58,59). Following the development of USPIO-enhanced MR, it has been adopted for use in a variety of pelvic malignancies (60), as well as it has been studied in bladder cancer. However, a high rate of adverse events with USPIO remain an important issue (46).
According to a study by Deserno et al. (44), who prospectively evaluated the effectiveness of USPIO-enhanced MR in preoperative nodal staging of 58 bladder cancer patients, it demonstrated accuracy, sensitivity, specificity and positive and negative predictive values (PPV and NPV) of 95%, 96%, 95%, 89% and 98% respectively, compared to precontrast values of 92%, 76%, 99%, 97%, and 91%, respectively. A clinical trial has been conducted by Thoeny et al. (61) in order to assess the value of combined USPIO-enhanced- and DW-MR (USPIO-DW-MR) imaging in the process of preoperative pelvic lymph node metastases detection, with both prostate cancer and bladder cancer patients being enrolled into the trial. The results of the trial have been published in three separate papers. In the early report by Thoeny et al. (43), imaging with USPIO-DW-MR was associated with a 92% rate of correct diagnosis (24 out of 26 lymph nodes assessed), with the only two LNs missed being micrometastatic. Later, after 2,993 lymph nodes of 75 patients have been analyzed, Triantafyllou et al. (42) reported USPIO-DW-MR imaging to demonstrate sensitivity, specificity, PPV, NPV and diagnostic accuracy of 55.0–58.3%, 85.5–83.0%, 57.9–58.0%, 83.9–84.4% and 77.3–76.4%, respectively, with the majority of missed metastases being smaller than 5 mm in short axis diameter. Based on the same trial, Birkhäuser et al. (41) reported a per-patient sensitivity and specificity for detection of LNI of 65–75% and 93–96%, respectively, and sensitivity and specificity per pelvic side 58–67% and 94–97% respectively.
Positron-emission tomography (PET)
Preoperative staging (Table 4)
Full table
18F-fludeoxyglucose-PET (FDG-PET) has been long proposed as a possible imaging modality for preoperative staging of patients in whom radical cystectomy for bladder cancer is considered (Figure 3). This has been, however, confronted with conflicting viewpoints in the reviewed literature.
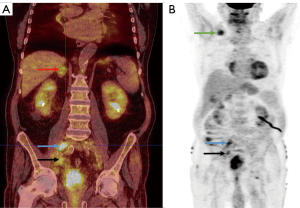
An early [2005] prospective study by Drieskens et al. (68) on 55 patients showed high concordance between FDG-PET/CT and CT alone results regarding sensitivity for lymph node metastasis detection, which was later confirmed by Swinnen et al. (33) who in 2010 reported similar results of a prospective study on 51 patients and concluded that FDG-PET/CT provides no real advantage in locoregional lymph node staging in bladder cancer. However, a similar prospective study by Lodde et al. (32), published later that year, revealed that FDG-PET/CT may be in fact more sensitive than CT in detecting lymph node metastases (sensitivity 57% vs. 33%). Other studies, published between 2009 and 2014, argued in favor of using FDG-PET/CT as a preoperative staging tool in bladder cancer patients. Kibel et al. (67), as well as Apolo et al. (31), who prospectively studied 43 and 57 (respectively) patients, reported both high sensitivity and specificity of FDG-PET/CT in detecting LNI (70% and 94% respectively for Kibel et al. and 92% and 81% for Apolo et al.). Hitier-Berhoult et al. (29) prospectively compared FDG-PET/CT and CT alone in 52 patients and reported significantly higher sensitivity of FDG-PET/CT in evaluating LN status for staging purposes, concluding that FDG-PET/CT is more reliable than CT alone. A bigger (n=102) study by Rouanne et al. (65) also showed high sensitivity and specificity values for FDG-PET/CT, which led to a conclusion pointing out improved diagnostic efficacy of FDG-PET/CT for lymph node staging.
However, later studies, which compared FDG-PET with CT showed less favorable results. Goodfellow et al. (27) in their study on 233 bladder cancer patients, published in 2014, showed only a small benefit in detecting lymph node metastases outside the pelvis when compared to CT (sensitivity 69% vs. 41% respectively), which the authors considered not to be enough to justify the use of preoperative FDG-PET outside of a proposed selected group of patients. Recently, Aljabery et al. (26) [2015] and Pichler et al. (23) [2017], based on the results of their studies both concluded that FDG-PET provided no advantage over CT in detection of metastatic lymph nodes in bladder cancer.
A novel study by Vind-Kezunovic et al. (63) was published in 2017, in which the authors evaluated the maximum standard uptake value (SUVmax) on FDG-PET scans. Based on data prospectively collected from 131 patients, the authors revealed that at SUVmax >2 FDG-PET scan sensitivity and specificity values in detecting metastatic LNs were 79.4% and 66.5%, respectively, while at SUVmax >4 FDG-PET showed sensitivity of 61.8% and specificity of 84.5%. The authors concluded that at higher SUVmax (>4) FDG-PET scans can aid in differentiation between patients with a poor prognosis.
The data regarding the use of FDG-PET/CT for the purpose of preoperative bladder cancer staging was recently meta-analyzed by Ha et al. (62) The meta-analysis included 14 studies, all of which are reviewed in this article (62,63,67-78); and revealed a relatively low pooled sensitivity of 57% and high pooled specificity of 92% of FDG-PET in preoperative detection of metastatic LNs. The authors pointed out the substantial heterogeneity of the studies in terms of the estimates of sensitivity and specificity, which, according to their analysis, could be explained both by diverse methodology between different studies and major baseline differences between patients.
Postoperative staging (Table 5)
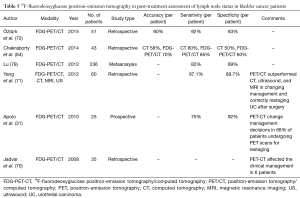
Full table
Postoperative nodal recurrence of urothelial carcinoma is associated with a poor prognosis (69). Although no therapy has been proven to significantly prolong survival in these patients, early and precise detection of disease recurrence may be of substantial significance in terms of selecting the right management strategy.
The number of studies evaluating the role of PET/CT in postoperative restaging of urothelial carcinoma patients is scant. However, the literature available suggests that this modality may be of significant value in terms of detecting nodal recurrence in bladder cancer. In an early [2008] study by Jadvar et al. (70), who retrospectively analyzed data from 35 patients, PET/CT was reported to be two times more sensitive than CT in detecting metastatic lymph nodes. Moreover, the information provided with PET/CT affected the clinical management in 6 patients (17% of-total), mainly by prompting additional therapy. This data is consistent with the conclusions of Apolo et al. (31), who also stated that according to their study results PET/CT scans changed the postoperative management of 68% BC patients. In a study by Yang et al. (71) PET/CT demonstrated sensitivity of 87.1% and specificity of 89.7% regarding the detection of LN metastases in BC recurrence, by which it outperformed CT, MRI, and ultrasound, and was noted to affect management plans. Recently [2015] Öztürk et al. (72) reported good outcomes of using PET/CT in identifying post-cystectomy metastases of BC, with sensitivity, specificity, PPV and NPV of 92%, 83%, 94%, 77%, respectively.
Apart from the above, according to the study by Giannatempo et al. (73), FDG-PET may be useful in the assessment of treatment response in metastatic urothelial carcinoma treated with chemotherapy alone, with a survival advantage reported in those with favorable response on FDG-PET imaging after two cycles of first-line chemotherapy.
Novel radiotracers (Table 6)
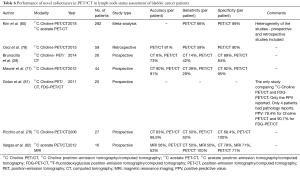
Full table
For the purpose of cancer staging, 18F-fludeoxyglucose (FDG) has been traditionally used as the radiotracer of choice. Besides, several other compounds have been employed for use in oncology, yielding varying results, with 11C-methionine, 11C-acetate, and 11C-choline being most widely used.
We found no essential research data regarding the use of 11C-methionine for the purpose of metastases detection in urothelial carcinoma patients among the results of our database search queries. This could be explained by the fact that the low specificity of 11C-methionine and increased background uptake make this radiotracer of very limited value in terms of precise urothelial cancer staging (74,75).
The value of the radiotracer 11C-choline in detecting metastases in cases of urothelial carcinoma has been assessed in several small studies on patients diagnosed with urothelial BC and referred for radical cystectomy with pelvic LN. In a prospective study on 27 patients by Picchio et al. (76), 11C-choline-PET/CT demonstrated 62% sensitivity and 100% specificity regarding the preoperative LN metastases detection. In a study by Brunocilla et al. (28), who compared preoperative 11C-choline-PET/CT to contrast-enhanced CT in 26 BC patients, 11C-choline-PET/CT was shown to have much greater sensitivity than CT, while demonstrating similar specificity. On the other hand, in a prospective study on 44 patients by Maurer et al. (77), 11C-choline PET/CT was found not to be able to improve preoperative diagnostic efficacy compared with conventional CT alone. Ceci et al. (78), who retrospectively analyzed data from 59 patients diagnosed with BC, making it the largest study on the utility of 11C-choline-PET up to date, reported sensitivity of 59%, specificity of 90%, positive predictive value of 71%, negative predictive value 84% and accuracy of 81% for nodal staging with 11C-choline-PET/CT.
Two studies concerning the use of 11C-acetate were included into this review, both being conducted in the same institution. Schöder et al. (83), in a study on 17 patients, reported results indicating 100% sensitivity and 87% specificity of 11C-acetate-PET/CT regarding correct identification of metastatic LN, with a significant rate of false positive uptake in LNs being secondary to inflammatory response due to prior intravesical chemotherapy. In a study by Vargas et al. (82)., who prospectively collected data from 16 patients referred for radical cystectomy with pelvic lymph node dissection, 11C-acetate-PET/CT, while demonstrating 100% sensitivity and 71% specificity for nodal staging, was concluded to display similar levels of accuracy with MRI and CT.
PET/MRI
PET/MR is a hybrid imaging modality that combines the ability of PET to delineate biochemical or physiologic phenomena with the high anatomic resolution of MR imaging. An inestimable advantage of PET/MR over PET/CT is the much lower dose of radiation (84). Yet, the evidence-based literature regarding the utility of PET/MR in nodal or metastatic staging of urothelial carcinoma is very scant. The only article we included into the review was a recent prospective pilot study by Rosenkrantz et al. (85), who prospectively compared the diagnostic performance of FDG-simultaneous PET/MRI and MRI alone. According to the study results, PET/MRI exhibited greater accuracy for detection of metastatic pelvic LNs (95% vs. 76% of MRI) and non-nodal pelvic malignancy (100% vs. 91%), and was concluded to have helped to appropriately determine the level of suspicion for equivocal findings on MRI alone.
Discussion
Increasing the accuracy of imaging studies for lymph node status evaluation in urothelial carcinoma patients is essential for establishing appropriate treatment strategies. While low sensitivity for detection of metastatic lymph nodes is associated with understaging of the disease, a decrease in specificity leads to an increased rate of overstaging. This is of clinical significance both at primary staging, where an understaged patient may be unnecessarily exposed to severe complications of surgical management or an overstaged patient may be wrongly disqualified from potentially curative treatment, and at posttreatment staging, or evaluation of treatment response, where overstaging may lead to further purposeless management or understaging may result in not applying necessary salvage treatment.
As previously mentioned in this article, CT is still recognized as the gold-standard imaging modality for nodal staging of urothelial carcinoma patients. However, given its low accuracy and sensitivity values for detecting lymph node metastases, this may be considered controversial. As shown in this review, despite relatively high specificity values of CT scans, usually exceeding 90%, no study reported its sensitivity to be greater than 46% (on a per-patient analysis and using standard cut-off values). Invasive urothelial carcinoma is a malignant disease associated with a high metastatic potential and thus an already clinically significant metastatic tumor in a lymph node may not be large enough to make the LN short-axis diameter exceed the cut-off value (23,30,31). Unfortunately, CT is not able to evaluate other lymph node features than its size or shape. Thus, in order to decrease the rate of false-negative rates in cases of small LN metastases, the only option would be to lower the short-axis diameter threshold, at the expense of specificity. However, apart from the article by Li et al., who actually suggested such an approach, no other study regarding this issue has been included into this review. In light of the above mentioned limitations of CT in regards to nodal status evaluation in urothelial cancer, the simplicity, cost-effectiveness and easy accessibility of this method appear to be its only main advantages (86,87). The rush towards developing or establishing a more accurate imaging modality is reflected by the fact that for the purpose of this review almost all of the available data regarding the diagnostic performance of CT has been abstracted from studies evaluating other modalities, with CT being used for comparison purposes only.
Magnetic resonance, which due to its increased accuracy has been adopted as the diagnostic modality of choice for nodal status evaluation in several other malignancies (52-56), is a method of imaging characterized with more diverse capabilities. As previously mentioned in this article, MR is able not only to measure the size and shape of a lymph node, but also to evaluate for detailed changes in its anatomy suggestive of metastatic involvement, as well as to assess the precise density of a tissue, or to dynamically visualize its function. Table 2 shows that the sensitivity value of MR regarding the detection of metastatic lymph nodes in urothelial cancer may be even as high as 88% (with concomitant specificity of 75%), but also as low as 40.7% (with concomitant specificity of 91.5%), the latter being similar to the diagnostic performance of CT. The marked variations between the results of different studies may be explained by their significant heterogeneity, regarding not only the character of the specific method studied, i.e., DW-MR or DCE-MR, but also to the strength of the magnetic field used. However, the results of the studies included into this review tend to suggest that the use of DW-MR for urothelial carcinoma nodal staging may yield relatively high sensitivity values, with no significant deterioration in terms specificity, and thus its use may result in lower rates of understaging compared to the golden-standard of CT. Yet, the amount of clinical evidence that would suggest this conclusion is scant and more research involving a precise imaging protocol is needed. Moreover, reproducibility of study results could be hindered by the marked heterogeneity between different MR scanners used in health-care facilities (35).
The diagnostic performance of MRI in detection of lymph node metastases in urothelial carcinoma patients can be further improved by the use of USPIO as the contrast agent. The results of the studies quoted in this review demonstrate impressively high sensitivity and specificity values of USPIO-MRI in regard to lymph node status evaluation. However, in our opinion, a study on 58 patients and a clinical trial with 75 patients do not serve as sufficient evidence to justify routine use of USPIO-MRI in urothelial carcinoma patients, especially given the significant risk of possible adverse effects associated with the use of this method (41,44). Moreover, the above studies demonstrated interestingly high diagnostic performance of pre-contrast DW-MRI alone. Although the influence of contrast enhancement appeared to be significant, the high sensitivity and specificity values of USPIO-MRI reported by those studies might be also explained by the accuracy of DW-MRI alone, which could serve as another evidence of the high potential of DW-MRI in terms of accurate nodal staging of urothelial carcinoma patients.
The ability of PET to evaluate the metabolic activity of investigated regions makes it a considerably distinct imaging modality in regard to nodal staging of malignant diseases. As the tridimensional map of highly-metabolic areas requires precise anatomic imaging for guiding purposes, a PET study is most commonly performed concomitantly with a CT scan (PET/CT). Thus PET imaging may be considered an essential adjunct to organ morphology assessment provided by CT, given the potential capability of PET to increase the sensitivity of CT by pointing out highly-metabolic metastatic LNs, otherwise not large enough to exceed the short-axis diameter cut-off value. Although the sensitivity of PET/CT is in fact negatively affected by small metastatic lesions not being able to reach threshold metabolic activity and there are studies presented in this review that based on their results doubt the potential of PET to increase the sensitivity of CT for primary lymph node staging of urothelial carcinoma, yet a substantial portion of the articles, including the recent meta-analysis by Li et al., may be considered suggestive of an actually high diagnostic performance of PET/CT compared to CT alone. However, as pointed out by the meta-analysis, there has been marked heterogeneity between the studies reviewed and thus further research using a uniform protocol is warranted in order to evaluate or establish a possibly superior role of PET/CT in the process of preoperative staging of urothelial carcinoma patients.
Apart from primary staging, the evidence-based literature, as presented in this review, also suggests a significant role of PET/CT at posttreatment staging, or evaluation of treatment response. An important advantage of PET is the ability to accurately differentiate between viable recurrences and treatment-induced changes of the LN morphology (88). Kollberg et al. (89), who investigated the results of PET/CT scans performed before and after neoadjuvant chemotherapy in 50 bladder cancer patients, reported PET/CT to demonstrate an 86% rate of correct prediction of the histological nodal chemotherapy response (9). As shown in this review, the few studies which evaluated the role of PET/CT in the process of nodal restaging, have shown relatively high sensitivity and specificity values of posttreatment PET/CT, as well as demonstrated its ability to influence the clinical decision making process and to change individual treatment plans. Thus, the authors of this article would like to emphasize the need for further research regarding this issue, given the promising character of the up-to-date reports.
While the majority of studies which evaluated the diagnostic performance of PET/CT used FDG as the radiotracer, the role of other compounds in the process of nodal staging of urothelial carcinoma has been assessed as well. While no study reliably compared 11C-choline-PET/CT or 11C-acetate-PET/CT versus FDG-PET/CT in terms of detecting metastatic LNs in urothelial carcinoma patients, the sensitivity and specificity rates of PET/CT scans using these novel radiotracers usually did not reach the values demonstrated by FDG-PET/CT, which was particularly noticeable for sensitivity, as presented in Table 6. However, a shortcoming of FDG is its renal route of excretion, which results in accumulation of the radiotracer in the urinary bladder. This may cause interference with visualization of adjacent locoregional lymph nodes, as the metabolic activity of lymph nodes is usually not high enough to make the radiotracer visible through the bladder content (90,91). While administration of diuretics may help to partially overcome these limitations, as reported by Nayak et al. (92), the use of 11C-choline or 11C-acetate may solve the problem entirely, as these compounds are associated with minimal urinary excretion. However, a significant issue limiting the accessibility of 11C-choline-PET is the radiotracer half-life of only 20 minutes, making it very difficult to perform the scan in a health-care facility without an onsite cyclotron available (90).
A relatively novel approach consists in combining PET with MRI instead of CT, which may be beneficial not only given the reduction in the dose of ionizing radiation (85), but also due to possible sensitivity boost provided by the earlier mentioned advantages of MR imaging. However, the pilot character of the only evidence-based data available so far, despite being interestingly optimistic, as presented in this review, makes it premature to draw any conclusions regarding the use of PET/MRI for the purpose of lymph node status evaluation in urothelial carcinoma patients. Moreover, the significant costs of PET/MRI (86), as well as low accessibility of the scanners (91), would definitely hinder implementation of this imaging modality into clinical practice. Obviously, the combination of advantages of PET and MR imaging could be also achieved by performing both PET/CT and MRI scans separately with subsequent cognitive fusion of results, however, we found no studies regarding this issue in the up-to-date literature.
It is worth mentioning, that the vast majority of studies listed among the results of the database search performed for the purpose of this systematic review concerned PET, which may be recognized as a reflection of current trends in clinical research. According to the authors of this article, establishment of conclusive evidence regarding the role of PET in the process of lymph node status evaluation in urothelial carcinoma patients is only a question of time.
An underinvestigated field of research in terms of metastatic lymph node detection in urothelial carcinoma is the so-called targeted imaging, which utilizes the ability of radiotracer-and-ligand complex to specifically bind to particular cancer-affected tissues. While, as an example, 99mTc-TSHR analogue has been studied for its potential role in detecting metastases of poorly differentiated metastatic thyroid cancer (93), or prostate-specific membrane antigen (PSMA)-PET (94,95) has been shown to improve the accuracy of lymph node status evaluation in prostate cancer, no method specific for urothelial carcinoma has been definitely developed up to date, despite intense ongoing efforts (96).
The authors of this review recognize that an important factor influencing the accuracy of lymph node status evaluation, namely the inter-reader variability, has not been systematically studied for urothelial carcinoma. Although few of the studies included into this review did group their results per reader, this data is insufficient to draw conclusions regarding this issue.
Conclusions
We have presented a systematic review of recent studies that evaluate the diagnostic performance of different imaging modalities in regards to detection of lymph node metastases in urothelial carcinoma patients. An explicit trend towards replacing CT with other methods of imaging can be observed in the current literature, as the limitations of CT, resulting primarily in its low sensitivity, are widely acknowledged. Although the up-to-date literature regarding the role of PET/CT, or MR imaging, or the fusion of both, may be recognized as promising, further research involving uniform protocols is required in order to establish one of those methods as the imaging modality of choice, or at least to recommend its routine use in selected group of patients. This may be in fact achieved relatively soon, as the amount of evidence is rapidly growing. Until then, given the uniqueness of every cancer patient, the authors believe that this review may appear to be helpful in individual decision making processes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ploeg M, Aben KKH, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol 2009;27:289-93. [Crossref] [PubMed]
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5; discussion 475-7.
- Kluth LA, Black PC, Bochner BH, et al. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur Urol 2015;68:238-53. [Crossref] [PubMed]
- Gontero P, Sylvester R, Pisano F, et al. Prognostic Factors and Risk Groups in T1G3 Non-Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Guerin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur Urol 2015;67:74-82. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Spiess PE, Agarwal N, Bangs R, et al. Bladder Cancer, Version 5.2017. J Natl Compr Canc Netw 2017;15:1240-67. [Crossref] [PubMed]
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol 2000;163:1693-6. [Crossref] [PubMed]
- Amin MB, Smith SC, Reuter VE, et al. Update for the practicing pathologist: The International Consultation On Urologic Disease-European association of urology consultation on bladder cancer. Mod Pathol 2015;28:612-30. [Crossref] [PubMed]
- Fonteyne V, Ost P, Bellmunt J, et al. Curative Treatment for Muscle Invasive Bladder Cancer in Elderly Patients: A Systematic Review. Eur Urol 2018;73:40-50. [Crossref] [PubMed]
- Noon AP, Albertsen PC, Thomas F, et al. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer 2013;108:1534-40. [Crossref] [PubMed]
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75. [Crossref] [PubMed]
- James ND, Hussain SA, Hall E, et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N Engl J Med 2012;366:1477-88. [Crossref] [PubMed]
- Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol 2017;71:952-60. [Crossref] [PubMed]
- Nishiyama H, Habuchi T, Watanabe J, et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: A survey including 1131 patients treated during 1990-2000 in Japan. Eur Urol 2004;45:176-81. [Crossref] [PubMed]
- Galsky MD, Stensland K, Sfakianos JP, et al. Comparative Effectiveness of Treatment Strategies for Bladder Cancer With Clinical Evidence of Regional Lymph Node Involvement. J Clin Oncol 2016;34:2627-35. [Crossref] [PubMed]
- Moschini M, Mattei A, Cornelius J, et al. Surgical treatment for clinical node-positive bladder cancer patients treated with radical cystectomy without neoadjuvant chemotherapy. World J Urol 2018;36:639-44. [Crossref] [PubMed]
- Ho PL, Willis DL, Patil J, et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: The MD Anderson Cancer Center Experience. Urol Oncol 2016;34:59.e1-8. [Crossref] [PubMed]
- Zargar-Shoshtari K, Zargar H, Lotan Y, et al. A Multi-Institutional Analysis of Outcomes of Patients with Clinically Node Positive Urothelial Bladder Cancer Treated with Induction Chemotherapy and Radical Cystectomy. J Urol 2016;195:53-9. [Crossref] [PubMed]
- Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: Results of a prospective multicenter study. J Urol 2004;171:139-44. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Li Y, Diao FY, Shi SY, et al. Computed tomography and magnetic resonance imaging evaluation of pelvic lymph node metastasis in bladder cancer. Chin J Cancer 2018;37:3. [Crossref] [PubMed]
- Pichler R, De Zordo T, Fritz J, et al. Pelvic Lymph Node Staging by Combined F-18-FDG-PET/CT Imaging in Bladder Cancer Prior to Radical Cystectomy. Clin Genitourin Cancer 2017;15:e387-e395. [Crossref] [PubMed]
- Horn T, Zahel T, Adt N, et al. Evaluation of Computed Tomography for Lymph Node Staging in Bladder Cancer Prior to Radical Cystectomy. Urol Int 2016;96:51-6. [Crossref] [PubMed]
- Jeong IG, Hong S, You D, et al. FDG PET-CT for Lymph Node Staging of Bladder Cancer: A Prospective Study of Patients with Extended Pelvic Lymphadenectomy. Ann Surg Oncol 2015;22:3150-6. [Crossref] [PubMed]
- Aljabery F, Lindblom G, Skoog S, et al. PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urol 2015;15:87. [Crossref] [PubMed]
- Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int 2014;114:389-95. [PubMed]
- Brunocilla E, Ceci F, Schiavina R, et al. Diagnostic Accuracy of C-11-Choline PET/CT in Preoperative Lymph Node Staging of Bladder Cancer. Clin Nucl Med 2014;39:e308-e312. [Crossref] [PubMed]
- Hitier-Berthault M, Ansquer C, Branchereau J, et al. F-18-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: A prospective study. Int J Urol 2013;20:788-96. [Crossref] [PubMed]
- Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol 2012;30:827-31. [Crossref] [PubMed]
- Apolo AB, Riches J, Schoeder H, et al. Clinical Value of Fluorine-18 2-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography in Bladder Cancer. J Clin Oncol 2010;28:3973-8. [Crossref] [PubMed]
- Lodde M, Lacombe L, Friede J, et al. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int 2010;106:658-63. [Crossref] [PubMed]
- Swinnen G, Maes A, Pottel H, et al. FDG-PET/CT for the Preoperative Lymph Node Staging of Invasive Bladder Cancer. Eur Urol 2010;57:641-7. [Crossref] [PubMed]
- Barentsz JO, Engelbrecht MR, Witjes JA, et al. MR imaging of the male pelvis. Eur Radiol 1999;9:1722-36. [Crossref] [PubMed]
- Woo S, Suh CH, Kim SY, et al. The Diagnostic Performance of MRI for Detection of Lymph Node Metastasis in Bladder and Prostate Cancer: An Updated Systematic Review and Diagnostic Meta-Analysis. AJR Am J Roentgenol 2018;210:W95-W109. [Crossref] [PubMed]
- Lin WC, Chen JH. Pitfalls and Limitations of Diffusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Urinary Bladder Cancer. Transl Oncol 2015;8:217-30. [Crossref] [PubMed]
- Wollin DA, Deng FM, Huang WC, et al. Conventional and diffusion-weighted MRI features in diagnosis of metastatic lymphadenopathy in bladder cancer. Can J Urol 2014;21:7454-9. [PubMed]
- Liedberg F, Bendahl PO, Davidsson T, et al. Preoperative staging of locally advanced bladder cancer before radical cystectomy using 3 tesla magnetic resonance imaging with a standardized protocol. Scand J Urol 2013;47:108-12. [Crossref] [PubMed]
- Daneshmand S, Ahmadi H, Huynh LN, et al. Preoperative Staging of Invasive Bladder Cancer With Dynamic Gadolinium-enhanced Magnetic Resonance Imaging: Results From a Prospective Study. Urology 2012;80:1313-8. [Crossref] [PubMed]
- Papalia R, Simone G, Grasso R, et al. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int 2012;109:1031-6. [Crossref] [PubMed]
- Birkhäuser FD, Studer UE, Froehlich JM, et al. Combined Ultrasmall Superparamagnetic Particles of Iron Oxide-Enhanced and Diffusion-weighted Magnetic Resonance Imaging Facilitates Detection of Metastases in Normal-sized Pelvic Lymph Nodes of Patients with Bladder and Prostate Cancer. Eur Urol 2013;64:953-60. [Crossref] [PubMed]
- Triantafyllou M, Studer UE, Birkhaeuser FD, et al. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer 2013;49:616-24. [Crossref] [PubMed]
- Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined Ultrasmall Superparamagnetic Particles of Iron Oxide-Enhanced and Diffusion-Weighted Magnetic Resonance Imaging Reliably Detect Pelvic Lymph Node Metastases in Normal-Sized Nodes of Bladder and Prostate Cancer Patients. Eur Urol 2009;55:761-9. [Crossref] [PubMed]
- Deserno WM, Harisinghani MG, Taupitz M, et al. Urinary bladder cancer: Preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology 2004;233:449-56. [Crossref] [PubMed]
- Hafeez S, Huddart R. Advances in bladder cancer imaging. BMC Med 2013;11:104. [Crossref] [PubMed]
- Green DA, Durand M, Gumpeni N, et al. Role of magnetic resonance imaging in bladder cancer: current status and emerging techniques. BJU Int 2012;110:1463-70. [Crossref] [PubMed]
- Panebianco V, De Berardinis E, Barchetti G, et al. An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur Radiol 2017;27:3759-66. [Crossref] [PubMed]
- Panebianco V, Barchetti F, de Haas RJ, et al. Improving Staging in Bladder Cancer: The Increasing Role of Multiparametric Magnetic Resonance Imaging. Eur Urol Focus 2016;2:113-21. [Crossref] [PubMed]
- El-Assmy A, Abou-El-Ghar ME, Mosbah A, et al. Bladder tumour staging: comparison of diffusion- and T-2-weighted MR imaging. Eur Radiol 2009;19:1575-81. [Crossref] [PubMed]
- Kobayashi S, Koga F, Yoshida S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 2011;21:2178-86. [Crossref] [PubMed]
- Sevcenco S, Ponhold L, Heinz-Peer G, et al. Prospective evaluation of diffusion-weighted MRI of the bladder as a biomarker for prediction of bladder cancer aggressiveness. Urol Oncol 2014;32:1166-71. [Crossref] [PubMed]
- Holzapfel K, Gaa J, Schubert EC, et al. Value of diffusion-weighted MR imaging in the diagnosis of lymph node metastases in patients with cholangiocarcinoma. Abdom Radiol (NY) 2016;41:1937-41. [Crossref] [PubMed]
- He XQ, Wei LN. Diagnostic value of lymph node metastasis by diffusion-weighted magnetic resonance imaging in cervical cancer. J Cancer Res Ther 2016;12:77-83. [Crossref] [PubMed]
- Zhong J, Lu ZH, Xu L, et al. The Diagnostic Value of Cervical Lymph Node Metastasis in Head and Neck Squamous Carcinoma by Using Diffusion-Weighted Magnetic Resonance Imaging and Computed Tomography Perfusion. Biomed Res Int 2014;2014. [Crossref] [PubMed]
- Klerkx WM, Veldhuis WB, Spijkerboer AM, et al. The value of 3.0 Tesla diffusion-weighted MRI for pelvic nodal staging in patients with early stage cervical cancer. Eur J Cancer 2012;48:3414-21. [Crossref] [PubMed]
- Fornasa F, Nesoti MV, Bovo C, et al. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging 2012;36:858-64. [Crossref] [PubMed]
- Mir N, Sohaib SA, Collins D, et al. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol 2010;54:358-64. [Crossref] [PubMed]
- Tomlinson B, Lin TY, Dall'Era M, et al. Nanotechnology in bladder cancer: current state of development and clinical practice. Nanomedicine 2015;10:1189-201. [Crossref] [PubMed]
- Sankineni S, Brown AM, Fascelli M, et al. Lymph Node Staging in Prostate Cancer. Curr Urol Rep 2015;16:30. [Crossref] [PubMed]
- Atri M, Zhang Z, Marques H, et al. Utility of preoperative ferumoxtran-10 enhanced MRI to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN 6671/GOG 0233. J Clin Oncol 2011;29:abstr 5035.
- Thoeny HC, Froehlich JM, Triantafyllou M, et al. Metastases in Normal-sized Pelvic Lymph Nodes: Detection with Diffusion-weighted MR Imaging. Radiology 2014;273:125-35. [Crossref] [PubMed]
- Ha HK, Koo PJ, Kim SJ. Diagnostic Accuracy of F-18 FDG PET/CT for Preoperative Lymph Node Staging in Newly Diagnosed Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Oncology 2018;95:31-8. [Crossref] [PubMed]
- Vind-Kezunovic S, Bouchelouche K, Ipsen P, et al. Detection of Lymph Node Metastasis in Patients with Bladder Cancer using Maximum Standardised Uptake Value and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Results from a High-volume Centre Including Long-term Follow-up. Eur Urol Focus 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Soubra A, Hayward D, Dahm P, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol 2016;34:1229-37. [Crossref] [PubMed]
- Rouanne M, Girma A, Neuzillet Y, et al. Potential impact of F-18-FDG PET/CT on patients selection for neoadjuvant chemotherapy before radical cystectomy. Eur J Surg Oncol 2014;40:1724-30. [Crossref] [PubMed]
- Jensen TK, Holt P, Gerke O, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with F-18-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: Correlation with histopathology. Scand J Urol Nephrol 2011;45:122-8. [Crossref] [PubMed]
- Kibel AS, Dehdashti F, Katz MD, et al. Prospective Study of F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Staging of Muscle-Invasive Bladder Carcinoma. J Clin Oncol 2009;27:4314-20. [Crossref] [PubMed]
- Drieskens O, Oyen R, Van Poppel H, et al. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging 2005;32:1412-7. [Crossref] [PubMed]
- Salama A, Abdelmaksoud AM, Shawki A, et al. Outcome of Muscle-Invasive Urothelial Bladder Cancer After Radical Cystectomy. Clin Genitourin Cancer 2016;14:e43-e47. [Crossref] [PubMed]
- Jadvar H, Quan V, Henderson RW, et al. F-18 -Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol 2008;13:42-7. [Crossref] [PubMed]
- Yang Z, Pan L, Cheng J, et al. Clinical value of whole body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in the detection of metastatic bladder cancer. Int J Urol 2012;19:639-44. [Crossref] [PubMed]
- Öztürk H, Karapolat I. Efficacy of F-18-fluorodeoxyglucose-positron emission tomography/computed tomography in restaging muscle-invasive bladder cancer following radical cystectomy. Exp Ther Med 2015;9:717-24. [Crossref] [PubMed]
- Giannatempo P, Alessi A, Miceli R, et al. Interim Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography for Early Metabolic Assessment of Therapeutic Response to Chemotherapy for Metastatic Transitional Cell Carcinoma. Clin Genitourin Cancer 2014;12:433-9. [Crossref] [PubMed]
- Ahlström H, Malmstrom PU, Letocha H, et al. Positron emission tomography in the diagnosis and staging of urinary bladder cancer. Acta Radiologica 1996;37:180-5. [Crossref] [PubMed]
- Letocha H, Ahlstrom H, Malmstrom PU, et al. Positron emission tomography with l-methyl-c-11-methionine in the monitoring of therapy response in muscle-invasive transitional-cell carcinoma of the urinary-bladder. Br J Urol 1994;74:767-74. [Crossref] [PubMed]
- Picchio M, Treiber U, Beer AJ, et al. Value of C-11-choline PET and contrast-enhanced CT for staging of bladder cancer: Correlation with histopathologic findings. J Nucl Med 2006;47:938-44. [PubMed]
- Maurer T, Souvatzoglou M, Kuebler H, et al. Diagnostic Efficacy of 11C Choline Positron Emission Tomography/Computed Tomography Compared With Conventional Computed Tomography in Lymph Node Staging of Patients With Bladder Cancer Prior to Radical Cystectomy. Eur Urol 2012;61:1031-8. [Crossref] [PubMed]
- Ceci F, Bianchi L, Graziani T, et al. C-11-Choline PET/CT and Bladder Cancer Lymph Node Metastasis Assessment With Pathological Specimens as Reference Standard. Clin Nucl Med 2015;40:e124-e128. [Crossref] [PubMed]
- Lu YY, Chen JH, Liang JA, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur J Radiol 2012;81:2411-6. [Crossref] [PubMed]
- Kim SJ, Koo PJ, Pak K, et al. Diagnostic accuracy of C-11 choline and C-11 acetate for lymph node staging in patients with bladder cancer: a systematic review and meta-analysis. World J Urol 2018;36:331-40. [Crossref] [PubMed]
- Golan S, Sopov V, Baniel J, et al. Comparison of C-11-Choline With F-18-FDG in Positron Emission Tomography/Computerized Tomography for Staging Urothelial Carcinoma: A Prospective Study. J Urol 2011;186:436-41. [Crossref] [PubMed]
- Vargas HA, Akin O, Schoeder H, et al. Prospective evaluation of MRI, C-11-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012;81:4131-7. [Crossref] [PubMed]
- Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med 2004;34:274-92. [Crossref] [PubMed]
- Hirsch FW, Sattler B, Sorge I, et al. PET/MR in children. Initial clinical experience in paediatric oncology using an integrated PET/MR scanner. Pediatr Radiol. 2013;43:860-75. [Crossref] [PubMed]
- Rosenkrantz AB, Friedman KP, Ponzo F, et al. Prospective Pilot Study to Evaluate the Incremental Value of PET Information in Patients With Bladder Cancer Undergoing F-18-FDG Simultaneous PET/MRI. Clin Nucl Med 2017;42:e8-e15. [Crossref] [PubMed]
- Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in Bladder Cancer. J Cancer Sci Ther 2012;S14. [PubMed]
- Bagheri MH, Ahlman MA, Lindenberg L, et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urol Oncol 2017;35:473-91. [Crossref] [PubMed]
- Patil VV, Wang ZJ, Sollitto RA, et al. F-18-FDG PET/CT of Transitional Cell Carcinoma. AJR Am J Roentgenol 2009;193. [Crossref] [PubMed]
- Kollberg P, Almquist H, Blackberg M, et al. F-18 Fluorodeoxyglucose-positron emission tomography/computed tomography response evaluation can predict histological response at surgery after induction chemotherapy for oligometastatic bladder cancer. Scand J Urol 2017;51:308-13. [Crossref] [PubMed]
- Szydlo M, Jadwinski M, Chmura A, et al. Synthesis, isolation and purification of 11C-choline. Contemp Oncol (Pozn) 2016;20:229-36. [Crossref] [PubMed]
- Vandenberghe S, Marsden PK. PET-MRI: a review of challenges and solutions in the development of integrated multimodality imaging. Phys Med Biol 2015;60:R115-R154. [Crossref] [PubMed]
- Nayak B, Dogra PN, Naswa N, et al. Diuretic F-18-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging 2013;40:386-93. [Crossref] [PubMed]
- Galli F, Manni I, Piaggio G, et al. Tc-99m-Labeled-rhTSH Analogue (TR1401) for Imaging Poorly Differentiated Metastatic Thyroid Cancer. Thyroid 2014;24:1297-308. [Crossref] [PubMed]
- Maurer T, Eiber M, Schwaiger M, et al. Current use of PSMA, PET in prostate cancer management. Nat Rev Urol 2016;13:226-35. [Crossref] [PubMed]
- Mena E, Lindenberg ML, Shih JH, et al. Clinical impact of PSMA-based F-18-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging 2018;45:4-11. [Crossref] [PubMed]
- Katsila T, Liontos M, Patrinos GP, et al. The New Age of -omics in Urothelial Cancer, Re-wording Its Diagnosis and Treatment. Ebiomedicine 2018;28:43-50. [Crossref] [PubMed]


