
Kidney cancer biobanking: considerations for a single institutional biorepository
Introduction
Kidney cancer is characterised by a wide spectrum of histological morphology and clinical behavior, and is most frequently managed with surgical resection. With healthcare moving towards personalized medicine, there is a call for improved clinical decision-making tools and for disease-specific therapies in the context of kidney cancer management. One of the current challenges in management is distinguishing aggressive histological variant cancers, such as clear cell (cc) renal cell carcinoma (RCC) from indolent subtypes of RCC. Research investigating differences between classical variant ccRCC and other RCC subtypes has historically been limited, due to low sample size availability in the non-ccRCC category. The aim was to establish a reliable bioresource, called the Kidney Cancer Biobank, containing high-quality specimens and corresponding clinical information, to promote ongoing research into ccRCC and non-ccRCC kidney cancer subtypes, and ultimately improve management paradigms for kidney cancer. This brief report outlines considerations for high quality institutional biobanking.
RCC accounts for >90% of kidney cancer, with the most common histological variant of RCC being ccRCC. This subtype represents 70% of RCC, however more than 15 histological subtypes of RCC are currently recognised (1), and emerging genomic research suggests the likelihood of sub-classification of subtypes (2). Large genomic and molecular profiling efforts, as produced by The Cancer Genome Atlas (TCGA), contain information on the three most common subtypes of RCC: ccRCC (3), papillary RCC (2) and chromophobe RCC (4). As more distinct subtypes of RCC are recognised, research relies on high-quality specimens to facilitate RCC subtype profiling.
The Kidney Cancer Biobank, housed at the Translational Research Institute in Brisbane, Australia, was developed to facilitate future research into kidney cancer. Since its establishment in 2013, the Kidney Cancer Biobank has evolved into a robust clinical research tool that contains high-quality patient tissue and comprehensive clinical data from more than 330 participants. Based on projected estimates, the Kidney Cancer Biobank will recruit between 700 and 1,000 participants by 2023 (5).
Ethics and governance
The Princess Alexandra Hospital (PAH) in Queensland, Australia is a part of the eHealth strategy established by the Queensland Government in 2006, which aimed to establish a digital care network for hospitals throughout Queensland (6). The PAH utilises an integrated electronic medical record (ieMR) system to provide up-to-date and longitudinal clinical information to health practitioners and researchers. This tool has allowed for comprehensive data collection, beyond a cross-sectional snapshot obtained directly from inpatients.
Ethics and governance are important considerations in biobanking. Institutional ethics approvals for this biobank were obtained by the Metro South Health Human Research Ethics Committee and the University of Queensland Human Research Ethics Committee, as described in a previous publication (5). The biobank utilises a broad consent model, whereby a clinical staff member discusses voluntary participation in the Kidney Cancer Biobank with eligible patients. Patients who are amenable are given a participant information sheet which provides details on the tissues and information to be collected through enrolment in the study, as well as information on how to withdraw from the study. Recently, approval for linkage of the biobank to the Queensland Cancer Registry was obtained through a Public Health Act application, which allowed for robust data on clinical outcomes to be recorded for participants.
The custodians of the Kidney Cancer Biobank have an ethical duty to ensure the privacy of participants and to maintain the confidentiality of their samples and data. To do this, participants are given a unique study code number which is linked to their hospital unit record number (URN). De-identified participant information and clinical data, in both soft and hard copy, are stored securely, and digital data is contained in a password-protected database, only accessible to authorised investigators and collaborators. In the event that the custodians of the Kidney Caner Biobank cease the ability to support the biobank, endeavors will be made be made to transfer custodianship to a local academic entity with the capacity to continue operation of the biobank. If a new custodian cannot be identified all banked material and associated clinical data will be destroyed and erased.
Banked material will only be destroyed when there is no scientific value to be gained from stored specimens and clinical data; in the event of the closure of the Kidney Cancer Biobank and no entity can be found to curate the collection; or upon the request of a donor.
Patient recruitment
All participants are recruited prospectively, through the PAH Department of Urology, through coordination between clinicians and research personnel. Eligibility criteria require that participants be scheduled for nephrectomy (radical or partial) for a suspected renal malignancy after a renal mass has been identified on imaging; patients must be >18 years of age; and they must give informed consent to use tissue and de-identified patient data for the purposes of kidney disease/cancer research. Participants are informed that they can withdraw from the study at any time, without consequence and that their tissue and clinical information will be removed from future projects and analysis.
Biofluid collection
All blood and urine samples are collected in the operating theatre, just prior to surgery, with patients routinely fasted. Up to 12 mL of blood are collected into Vacutainer K2 EDTA tubes (BD Bioscience) placed on ice and immediately transported to the laboratory for centrifugation at 1,200 g for 10 min at 4˚C. Plasma is aliquoted into screw cap 2D data-matrix coded tubes in 500 µL volumes (4–6 aliquots per patient). Plasma, buffy coat and erythrocytes are stored at −80 ˚C.
Fifteen to 20 mL of urine are collected from a Foley catheter bag into a 50 mL Falcon tube (BD Bioscience) placed on ice and transported to the laboratory. Urine is centrifuged at 1,200 g for 10 min at 4 °C and aliquoted into screw cap 2D data-matrix coded tubes in 500 µL volumes (16 aliquots per patient).
Tissue collection
For patients undergoing radical nephrectomy, the following tissue samples are taken: renal tumor from the peripheral edge of tumor (to avoid tumor necrosis); non-cancer renal cortical tissue taken distal to the tumor; core biopsy of tumor; and perinephric fat surrounding tumor. All collected tissue is in excess to the needs of pathology. Research personnel coordinate with clinical staff, in order to obtain fresh tissue specimens in the operating theatre, once the kidney has been excised. Tissue specimens are obtained using a sterile scalpel and forceps. For tissue collection a sample is excised from the area of interest (tumor, non-tumor, fat) and then sub-sectioned. This is to ensure that formalin-fixed paraffin-embedded (FFPE) samples are representative of banked fresh frozen tissue samples. Sample processing time for all tissues, from collection to storage, does not exceed 2 h.
Since its establishment in 2013, the Kidney Cancer biobank has undergone significant updates in the way specimens are stored, labelled and annotated (Table 1). This includes the use of 2D barcoded labels for specimens, and a tissue specimen collection sheet for annotation of anatomical and gross observations at time of collection. These changes were made to keep the Kidney Cancer Biobank current with other institutional biobanking efforts (7).
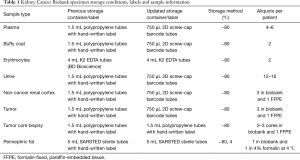
Full table
FFPE tissues and digital archive
The Kidney Cancer Biobank contains FFPE tissue samples of non-tumor renal cortex, renal tumor, and core biopsy material. FFPE blocks are sectioned into slides and routinely stained with hematoxylin and eosin. Slides are digitally scanned through the Microscopy Core Facility at the Translational Research Institute, using an Olympus Slide scanner VS120, and digital slides are archived in an Olyvia file format. FFPE blocks are stored long term, available for research use.
Current and future directions
While state-wide, government supported biobanking initiatives have been successful in some Australian states, such as New South Wales, a national biobanking initiative for Australia is not currently operating. Until national biobanking strategies are implemented, state-run and institutional biobanks with a clear scope, containing high-quality biospecimens and linked clinical data will continue to facilitate translational research. Moving forward, there is the possibility for collaboration with other institutional biobanks to bolster biospecimen availability. As a tertiary health care centre, the PAH is well positioned for continued recruitment of participants for the Kidney Cancer Biobank, which could become a biobanking hub in networked biobanks in Australia.
The histological variants of kidney cancer represented within the Kidney Cancer Biobank, as well as basic demographic data of participants are listed in Table 2. Currently, the Kidney Cancer Biobank is being used to facilitate a number of in-house research projects, including the CKD-TUNED study which is a longitudinal study looking at chronic kidney disease in patients undergoing surgical nephrectomy; a metabolomics study investigating metabolic changes in biofluids of patients with kidney cancer, using nuclear magnetic resonance spectroscopy; a translational metabolomics study that investigates metabolic signatures in nephrectomy tissue ex vivo and in vivo using magnetic resonance; and a study investigating glucose-regulated protein 78 (GRP78) as a tumor biomarker in perinephric adipose tissue.
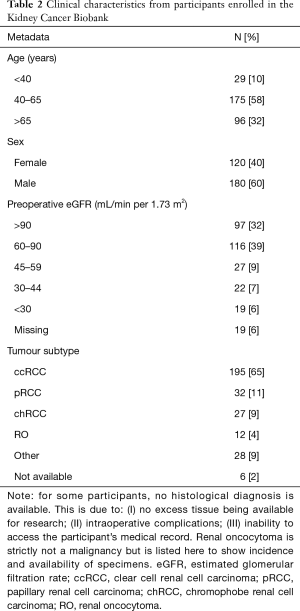
Full table
Conclusions
The establishment of reliable bioresources in the context of kidney cancer is especially important due to emerging histological variants of RCC, and a corresponding lack of disease-specific management options. Studies have demonstrated that subtypes of RCC have distinct genetic determinants, with a wide range of clinical behaviour and associated risk. Currently, mechanistic therapies and management strategies for non-ccRCC tumors are lacking. In the absence of national and international biobanking strategies, institutional biobanks continue to play a role in translational research projects. Successful institutional biobanking relies on biobanks having a clear scope; ethical stewardship and governance structures; maintenance of participant privacy, confidentiality, and ability to withdraw from the biobanking resource; and long-term specimen storage and labelling systems.
Acknowledgments
We would like to acknowledge Dr. Simon Wood, Director of Urology at the Princess Alexandra Hospital, Brisbane Queensland and our research colleagues at the Centre for Kidney Disease Research, University of Queensland. Dr. Keng Lim Ng, established the Kidney Cancer Biobank in 2013 as part of his PhD thesis. Dr. Hemamali Samaratunga is the participating uro-pathologist and Dr. Sonja Gustafson is the participating radiologist. We also acknowledge the involvement of the surgery, pathology and anaesthetic teams, and nursing staff, at the Princess Alexandra Hospital, who aid in sample collection and processing.
We gratefully acknowledge the generous donations of all participants in this biobangk.
This study is supported through grants from Metro South Hospital and Health Service. RJ Ellis is supported by an Australian Government Research Training Scholarship and a scholarship from the Royal Australasian College of Pathologists. SJ Del Vecchio is supported by an Australian Government Research Training Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016;374:135-45. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43-9. [Crossref] [PubMed]
- Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014;26:319-30. [Crossref] [PubMed]
- Ellis RJ, Del Vecchio SJ, Ng KL, et al. The Correlates of Kidney Dysfunction – Tumour Nephrectomy Database (CKD-TUNED) Study: Protocol for a Prospective Observational Study. Asian Pac J Cancer Prev 2017;18:3281-5. [PubMed]
- Shepherd T, Hain S. eHealth in Queensland: Progressing towards a Patient Centric, Networked Model of Care. Healthc Inform Res 2011;17:190-5. [Crossref] [PubMed]
- Owens EP, Hoy WE, Cameron A, et al. An Australian Chronic Kidney Disease Biobank to Support Future Research. Open J Bioresour 2018. In press.


