Exogenous testosterone: a preventable cause of male infertility
Introduction
In a recent survey of U.S. urologists, Ko et al. (1) observed that approximately 25% have treated low testosterone levels associated with male infertility with exogenous testosterone. Many physicians are unaware that testosterone replacement therapies (TRT) adversely affect spermatogenesis. Increasingly, the diagnosis of hypogonadism is being made in younger men, many of whom are of reproductive age. In 1970, less than 15% of all men fathering children were over 35. Today, this percentage has risen to almost 25%. There has been a notable increase in fatherhood even among men in the 50 to 54 age group (2). However, there is a lack of expert recommendations regarding hormone replacement therapy in men of reproductive age.
Case presentation
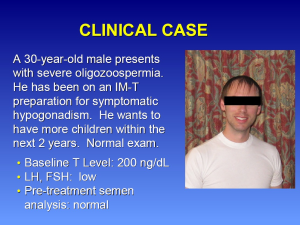
A 30-year-old male presents with severe oligozoospermia (Figure 1). He has been on an intramuscular testosterone (IM-T) preparation for the treatment of symptomatic hypogonadism. He desires to have more children within in the next two years and has a normal exam. His baseline testosterone level was 200 ng/dL. The luteinizing hormone (LH) and follicular-stimulating hormone (FSH) levels were low. The pre-treatment semen analysis was normal. For potential treatment options, the physician could advise that (I) he switch to a topical testosterone; (II) stop testosterone therapy; (III) change to clomiphene citrate; or (IV) add human chorionic gonadotropin (hCG) to his IM-T.
The best suggested answers are b and c (Figure 2). Option d may also be considered. Switching to a topical testosterone would still result in suppression of spermatogenesis. Similar effects on the HPG axis would be expected regardless of the route of administration. Stopping testosterone therapy is necessary to restore baseline HPG axis function and subsequent effects on spermatogenesis. Initiation of clomiphene citrate would address his symptomatic hypogonadism by increasing endogenous production of testosterone. Finally, there is a subset of men who do not want to consider cessation of testosterone therapy. For these men, addition of hCG to the IM-T has demonstrated beneficial effects on maintaining spermatogenesis.
Regarding the treatment of similar hypogonadal men of reproductive age, this AUA Plenary Session Presentation will review (I) the extent of the problem; (II) the mechanisms by which testosterone therapy impairs spermatogenesis and (III) therapeutic approaches to protect the testis.
The extent of the problem
Symptomatic hypogonadism is not uncommon (3). It is estimated that more than 6.5 million men in the U.S. will have symptomatic androgen deficiency by 2025 (4). Between the ages of 20 and 30 years, men experience a decline in testosterone and free testosterone levels by 0.4% and 1.3% per year, respectively (5). Indeed, Mulligan et al. (6) observed that roughly 39% of men over the age of 45 had low serum testosterone levels, defined as less than 300 ng/dL.
Testosterone therapies have been increasingly utilized in aging men, as well as in men of reproductive age. Compared to the 1970s men are fathering children at an older age. Combined with the maturation of the Baby Boomer population, it is anticipated that there may be a significant increase in hypogonadal, aging men desiring to father children. The treatment of hypogonadism requires symptoms, as well as low serum testosterone levels. With the recent introduction of several newer commercial testosterone preparations and an increased public awareness of androgen deficiency syndromes, use of hormone replacement therapies (HRT) has been increasing. Over the past five years there has been an increase in testosterone prescriptions by 170% (7). However, men desiring to maintain their reproductive potential may not be fully aware of the risks of exogenous testosterone therapy.
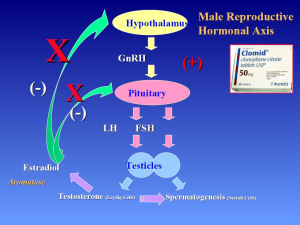
Testosterone users and health care professionals are often unaware that exogenous testosterone suppresses the hypothalamic-pituitary-gonadal axis and may result in infertility. Physicians need to educate their patients about the potentially deleterious effects exogenous testosterone can have on spermatogenesis and on fertility. Indeed, use of intramuscular testosterone has been investigated as a male contraceptive agent (8).
How testosterone inhibits spermatogenesis and fertility
Mechanism of action
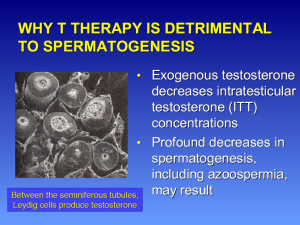
Testosterone inhibits both GnRH and gonadotropin secretion. Exogenous administration of synthetic testosterone results in negative feedback on the hypothalamic-pituitary axis, inhibiting GnRH, leading to inhibition of FSH and LH production. As a result, intratesticular testosterone levels (ITT) and overall testosterone production decrease. Exogenous testosterone therapies can suppress ITT production to such a degree that spermatogenesis can be dramatically compromised at ITT concentrations to less than 20 ng/mL, even resulting in azoospermia (9-11) (Figure 3).
Spermatogenesis recovery after discontinuation of exogenous testosterone
Recovery of spermatogenesis after discontinuation exogenous testosterone is generally promising. First, the hypogonadal male interested in fathering children should attempt cessation of use of exogenous testosterone. A study by the WHO Task Force evaluated 271 men who received 200 mg of testosterone enanthate weekly (12). After 6 months, 157 (65%) of the men were azoospermic with the mean time to azoospermia at 120 days. After 6 months of treatment, the patients entered the recovery phase. While 84% of men were able to achieve a sperm density >20 million/mL after a median of 3.7 months, only 46% of patients were able to achieve their baseline sperm density.
Mills and associates evaluated the recovery of spermatogenesis after exogenous testosterone administration in of 26 men with a recent history of anabolic steroid use (13). All discontinued exogenous testosterone usage and were treated with hCG 3,000 units IM every other day for a minimum of 3 months. Of the two men who remained azoospermic, one had insufficient follow-up and the other was suspected of continued anabolic steroid use. Men using intramuscular testosterone at the time of presentation recovered spermatogenesis in an average of 3.1 months. However, men receiving transdermal testosterone supplementation at the time of presentation took an average of 7.4 months. The authors concluded that impairment of fertility following TRT suppression is reversible and that the rate of sperm may be related to the delivery system.
The published literature represents the best available evidence to date regarding the recovery of spermatogenesis after testosterone supplementation. A significant problem is that literature assesses the use of testosterone therapy as a male contraceptive agent. This situation may not reflect the hypogonadal male seen in clinical practice. However, most men who discontinue T supplementation have a return of normal sperm production within one year. The consistency of spermatogenesis recovery in clinical practice may not be as predictable as in contraceptive studies.
Testosterone as male contraceptive
Pharmaceutical companies have tried to develop hormonal male contraceptives with the intent of causing a withdrawal of the gonadotropin support to the testis with resultant suppression of spermatogenesis and ITT (14). Testosterone has been studied alone, as well as in combination with progestrogens (15). Testosterone used as a contraceptive agent may be used to determine the recovery of spermatogenesis after cessation of therapy. This analysis regarding the application of contraceptive studies to hypogonadal males is problematic. First, the men studied in contraceptive trials were not hypogonadal and did not have concerns about fertility. Many of these men had normal baseline testosterone levels, as well as sperm production. Additionally, different testosterone preparations and doses were used compared to clinical formulations.
Testosterone undecanoate was administered at a dose of 500 mg monthly for 30 months in a study of ethnic Chinese men (16). Over a 24-month efficacy phase (855 men), a cumulative contraceptive failure rate of 1.1% per 100 men was reported. Failure to achieve azoospermia or severe oligozoospermia (<1×106 sperm/mL) was seen in 4.8%. Median time to onset of azoospermia or severe oligozoospermia was 108 days. Spermatogenesis returned to the normal fertile reference range in all but two participants. The median time to recovery of spermatogenesis calculated from the beginning of the recovery phase was 196 days. Importantly, spermatogenesis recovered to normal reference levels (sperm concentration ranging from 0 to 19×106/mL) in all but 17 participants who completed the 12-month recovery period, and 15 of those returned to normal reference levels at an extra 3-month follow-up visit. No long term data are available after the 2.5 year follow-up period.
An integrated, multivariate analysis of 30 studies was published by Liu et al. (8). The primary outcome was the time for the sperm concentration to recover to a threshold of 20 million/mL. Healthy eugonadal men aged 18-51 years were treated with androgens or androgens plus proestrogens. The median times for sperm to recover to thresholds of 20, 10, and 3 million per mL were 3.4, 3.0, and 2.5 months, respectively. Older age, Asian origin, shorter treatment duration, shorter-acting testosterone preparations, higher sperm concentrations at baseline, faster suppression of spermatogenesis, and lower LH levels at baseline were associated with higher rates of recovery (Figure 4). The contraceptive trials were in men of Chinese ethnicity and that comparison of findings to men of non-Chinese ethnicities may not be reliable.
The typical probability of recovery to 20 million per mL was 67% within 6 months, 90% within 12 months, 96% within 16 months, and 100% within 24 months (Table 1). This observed time to recovery may be helpful for counseling patients, but it should be cautioned that return of spermatogenesis may be prolonged for a small number of men. This study concluded that hormonal male contraceptive regimens show full reversibility within a predictable time course. A significant limitation of the published literature is a lack of pregnancy outcome data. Also, semen analysis data do not correlate with pregnancy outcomes and that none of the present literature addresses time to fecundity.
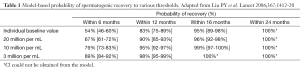
Full table
Treatment strategies
Exogenous testosterone use should be discouraged for hypogonadal men desiring to preserve their fertility. As seen in the contraceptive trials, restoration of baseline serum testosterone levels may be seen after discontinuation of testosterone therapy. An increased production of endogenous testosterone may be accomplished using strategies such as SERMs, hCG, and aromatase inhibitors (Figure 5). However, all of these methods, except for hCG injections, are considered off-label. It is important that any underlying causes of hypogonadism are identified.
Selective estrogen receptor modulators: clomiphene citrate
Clomiphene citrate (CC) is a selective estrogen receptor modulator (SERM) (17). This class of medications competitively binds to estrogen receptors on the hypothalamus and pituitary gland (Figure 6). Therefore, the pituitary sees less estrogen, and makes more LH, increasing testosterone production by the testes. Common dosing starts at 25 mg orally every other day with upward titration to 50 mg daily, as needed. Efficacy will be limited when LH and FSH levels are elevated, as seen in primary testis failure. Tamoxifen citrate is another SERM. Potential side effects include gynecomastia, weight gain, hypertension, cataracts, and acne.
CC has well-established efficacy in increasing serum testosterone levels (18,19). CC resulted in significant increases in testosterone levels from baseline in a study by Taylor and Levine, with increases similar to those with testosterone gel replacement therapy (TGRT) (20). Average post-treatment testosterone was 573 ng/dL (baseline 277 ng/dL) in the CC group and 553 ng/dL (baseline 221 ng/dL) in the TGRT group. CC represents a treatment option for men with hypogonadism, demonstrating biochemical and clinical efficacy with few side effects and lower costs than TGRT.
In a recent investigation, Katz and Mulhall from the Memorial Sloan-Kettering Cancer Center observed that long-term use of CC was safe and effective in improving serum testosterone levels to normal (4). In this moderately-sized analysis, eighty-six men aged 22- to 37-year-old men with hypogonadism (T levels <300 ng/dL) were evaluated and treated for a mean duration of 19 months. CC was started at 25 mg every other day and titrated to 50 mg every other day. Target testosterone level was 550 ng/dL. Significant improvements were reported in every area except for loss of height on the Androgen Deficiency in Aging Males (ADAM) questionnaire. Significant improvements were seen in libido, energy, life enjoyment, feeling sad/grumpy, and sports performance. This study demonstrated that CC is an effective and safe alternative to testosterone supplementation therapy in hypogonadal men with a medium range follow-up.
Human chorionic gonadotropin (hCG)
hCG is an LH analog that stimulates Leydig cell production of testosterone. ITT concentrations and serum testosterone levels are elevated with the use of exogenous hCG. Intramuscular injections 2 to 3 times per week at doses of 2,000 to 3,000 units for four months can initiate spermatogenesis for men with hypogonadotropic hypogonadism (21).
Spermatogenesis is maintained for shorter durations with hCG alone. In a case series by Nieschlag and Depenbusch, first hCG and human menopausal gonadotropin (hMG) were administered to 13 azoospermic men with hypogondotropic hypogonadism (22). Second, hCG was administered 500-2,500 IU hCG subcutaneously twice a week alone for up to two years ranging from 3 to 24 months. Sperm decreased gradually but was present after 12 months in all patients, except one who became azoospermic. Thus, FSH is necessary for maintenance of spermatogenesis.
Low doses of hCG are used to stimulate and maintain spermatogenesis. In a randomized trial by Roth et al., 37 normal men with the GnRH antagonists received one of four low doses of hCG: 0, 15, 60, 125 IU SC every other day or 7.5 g daily testosterone gel for 10 days (23). Steroid measurements were obtained from testicular fluid by percutaneous aspirations at baseline and after 10 days of treatment. ITT concentrations increased from 77 to 923 nmol/liter in the 0- and 125- IU groups, respectfully (P<0.001). Serum hCG was correlated significantly with both ITT and serum testosterone (P<0.001). The authors concluded that in normal men with experimental gonadotropin deficiency doses of hCG far lower than those used clinically increase ITT concentrations in a dose-dependent manner. The use of hCG may be beneficial in raising serum testosterone levels and preserving fertility, however the cost of hCG injection and its invasive administration can discourage its use.
hCG and testosterone
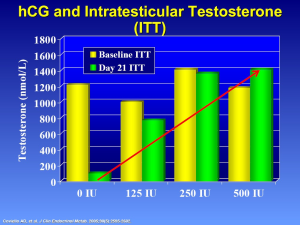
Intramuscular testosterone enanthate (200 mg/week) with low dose hCG can preserve ITT and serum testosterone levels (24). In a 3 week study of normal men, Coviello et al. administered low doses of hCG (0, 125, 250, or 500 IU every other day) and measured serum and ITT levels (Figure 7). The addition of low dose hCG maintained ITT levels while the administration of testosterone alone resulted in profound decreases in ITT concentration. Although serum T increased (80-fold higher) from baseline in all groups, ITT remained significantly higher in all four groups after treatment (24). Despite clinical doses of exogenous testosterone, low-dose hCG can maintain high levels of ITT.
Hsieh and Lipshultz demonstrated that low dose hCG (500 IU every other day) appears to maintain semen parameters in hypogonadal men on topical or intramuscular testosterone therapy (25). With a mean follow-up of 6 months, no patient became azoospermic during concomitant testosterone replacement and hCG therapy. While further study is required, concurrent testosterone replacement and human chorionic gonadotropin use may preserve fertility in hypogonadal males who desire fertility preservation while on testosterone replacement therapy.
Aromatase inhibitors (anastrozole and letrozole)
Aromatase, a cytochrome P-450 enzyme, is responsible for the conversion of testosterone to estradiol and is found in the testis, liver, brain, and adipose tissue. Estradiol inhibits gonadotropin secretion and may exert direct effects on ITT production. Their inhibitors function by blocking the conversion of androgens to estrogen, therefore increasing serum levels of LH, FSH and testosterone. Antiestrogens produce a very similar effect. Aromatase inhibitors have been used to improve male fertility and stimulate the production of sperm. In men with lower serum testosterone to estradiol ratios (<10) and in obese patients, aromatase inhibitors may have a greater benefit than antiestrogens.
Aromatase inhibitors have been used to treat men with Klinefelter’s syndrome for normalization of serum testosterone levels before microscopic testicular sperm extraction. They have also been used to treat men with idiopathic male infertility, men with lower serum testosterone to estradiol ratios (<10), and men with hypogonadism.
Adverse reactions include increases in blood pressure, rash, paresthesis, malaise, aches, peripheral edema, glossitis, anorexia, nausea/vomiting, and, rarely, alopecia that has been resolved spontaneously. Any form of therapy increasing serum testosterone levels, increases PSA levels, therefor these measurements would be beneficial for at-risk populations (26,27). The main concern associated with aromatase inhibitors is that long-term estrogen deficiency may lead to osteopenia or osteoporosis in men. In a recent study, after one year of anastrozol therapy in hypogonadal older men (mean age 60) showed a decrease in spinal BMD (28). Long-term potential effects of Aromatase inhibitors on bone health are a concern for physicians.
Conclusions
Exogenous testosterone therapy decreases sperm production and has detrimental effects on male fertility. However, after discontinuing testosterone supplementation, studies of hormonal contraception indicate that most men have a return of normal sperm production within 1 year. Exogenous testosterone use should be avoided in men desiring future fertility. Clomiphene citrate is an off-label, but well-tolerated and effective therapy for men desiring to preserve future potential fertility. hCG therapy with or without testosterone supplementation represents an alternative treatment. Currently, the use of aromatase inhibitors is not recommended based on the lack of long-term data. Published literature to date is still limited. In the future, this area of interest will be important, especially regarding data on pregnancy outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Presented at the 2013 AUA Plenary Session in San Diego, CA.
References
- Ko EY, Siddiqi K, Brannigan RE, et al. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol 2012;187:973-8. [PubMed]
- Lewis BH, Legato M, Fisch H. Medical implications of the male biological clock. JAMA 2006;296:2369-71. [PubMed]
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241-7. [PubMed]
- Katz DJ, Nabulsi O, Tal R, et al. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int 2012;110:573-8. [PubMed]
- Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737-45. [PubMed]
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762-9. [PubMed]
- Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med Wkly 2012;142:w13539. [PubMed]
- Liu PY, Swerdloff RS, Christenson PD, et al. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet 2006;367:1412-20. [PubMed]
- Zirkin BR, Santulli R, Awoniyi CA, et al. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 1989;124:3043-9. [PubMed]
- McLachlan RI, O’Donnell L, Meachem SJ, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl 2002;23:149-62. [PubMed]
- Weinbauer GF, Nieschlag E. Gonadotrophin-releasing hormone analogue-induced manipulation of testicular function in the monkey. Hum Reprod 1993;8 Suppl 2:45-50. [PubMed]
- Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 1990;336:955-9. [PubMed]
- Mills. American Urologic Association. J Urol 2008: Abstract #2461.
- Anderson RA, Wallace AM, Kicman AT, et al. Comparison between testosterone oenanthate-induced azoospermia and oligozoospermia in a male contraceptive study. IV. Suppression of endogenous testicular and adrenal androgens. Hum Reprod 1997;12:1657-62. [PubMed]
- Wang C, Leung A, Superlano L, et al. Oligozoospermia induced by exogenous testosterone is associated with normal functioning residual spermatozoa. Fertil Steril 1997;68:149-53. [PubMed]
- Gu Y, Liang X, Wu W, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab 2009;94:1910-5. [PubMed]
- Shelly W, Draper MW, Krishnan V, et al. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv 2008;63:163-81. [PubMed]
- Tenover JS, Bremner WJ. The effects of normal aging on the response of the pituitary-gonadal axis to chronic clomiphene administration in men. J Androl 1991;12:258-63. [PubMed]
- Guay AT, Bansal S, Heatley GJ. Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab 1995;80:3546-52. [PubMed]
- Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med 2010;7:269-76. [PubMed]
- Turek PJ, Williams RH, Gilbaugh JH 3rd, et al. The reversibility of anabolic steroid-induced azoospermia. J Urol 1995;153:1628-30. [PubMed]
- Depenbusch M, von Eckardstein S, Simoni M, et al. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol 2002;147:617-24. [PubMed]
- Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab 2010;95:3806-13. [PubMed]
- Coviello AD, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab 2005;90:2595-602. [PubMed]
- Burnett-Bowie SA, Roupenian KC, Dere ME, et al. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2008. [Epub ahead of print]. [PubMed]
- Hsieh TC, Pastuszak AW, Hwang K, et al. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 2013;189:647-50. [PubMed]
- Guay AT, Perez JB, Fitaihi WA, et al. Testosterone treatment in hypogonadal men: prostate-specific antigen level and risk of prostate cancer. Endocr Pract 2000;6:132-8. [PubMed]
- Burnett-Bowie SA, McKay EA, Lee H, et al. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 2009;94:4785-92. [PubMed]