
Challenges of kidney transplantation in HIV positive recipients
Introduction
Human immunodeficiency virus (HIV) infection is a well-established etiology of kidney disease and is characterized by nephrotic range proteinuria, followed by progressive renal failure (1-3). HIV infection can directly cause renal dysfunction in a variety of ways, including HIV-associated nephropathy (HIVAN), immune complex diseases, and thrombotic microangiopathy. HIVAN occurs in approximately 10% of patients with HIV (4) and is the third most common etiology of end-stage renal disease (ESRD) among African Americans aged 20–64 years (5,6). HIV can also indirectly cause kidney disease through the nephrotoxicity associated with antiretroviral therapy (ART) (e.g., tenofovir, indinavir) and antimicrobials used to treat opportunistic infections (e.g., trimethoprim-sulfamethoxazole, aminoglycosides, acyclovir, amphotericin, and foscarnet) (7,8). Despite the adverse effects of HIV infection on renal function, widespread use of highly active antiretroviral therapy (HAART) has decreased the number of patients requiring dialysis and has improved overall survival (9,10). HIV was once considered a contraindication to transplantation in the United States and Europe due to concerns about HIV disease progression in the setting of transplant immunosuppression (11-13). As a result, dialysis was believed to be the only solution for ESRD in HIV patients. However, kidney transplantation in the HAART era has shown improved overall survival compared to HIV patients on dialysis, and similar graft survival rates to HIV-negative patients (14,15). Due to these encouraging results, HIV-seropositivity is no longer a contraindication to renal transplantation at some centers (14,16,17). In this study, we review the most recent publications regarding the safety, efficacy, outcomes, and challenges of kidney transplantation in HIV positive recipients.
Methods
We performed a comprehensive literature search using the PubMed database from January 1st, 2000 to June 1st, 2018. For our search strategy, we used the combination of the Mesh terms “Kidney transplantation” and “HIV”. We limited our search to only include articles written in English.
History
The first series of kidney transplantation in HIV-positive patients occurred in 2003, which included 10 patients that were followed for a mean of 480 days (18). A standard post-transplantation immunosuppression regimen of prednisone, mycophenolate mofetil (MMF), and cyclosporine was used (18). At 3 months post-transplantation, all patients had undetectable plasma HIV-1 RNA levels, CD4 T-cell counts >200 cells/µL, and no history of opportunistic infections or neoplasms (18). However, half of the patients experienced acute rejection requiring treatment with antithymocyte globulin (ATG). At 1 year post-transplantation, HIV viral loads remained undetectable in all patients maintained with HAART, and CD4 counts remained stable in patients not treated for rejection (18). Later publications have reported similar results (19,20) with one study reporting normal renal function at 1 year post-transplantation in 3 HIV patients who underwent a living-related donor kidney transplantation with alemtuzumab preconditioning and steroid-free tacrolimus monotherapy (21).
Indications and patient selection for kidney transplantation in HIV-positive patients
In general, the indications for kidney transplantation in HIV-positive patients are the same as in HIV-negative patients. Patients with ESRD and well-controlled HIV, who are otherwise medically healthy, are the best candidates for kidney transplantation in this patient population (22). Groups in North America and Europe have worked to establish common transplant waiting list criteria for HIV-positive patients (23-26). However, the general consensus is that the recipient should have a CD4 count >200 cells/µL, an HIV viral load <50 HIV-1 RNA copies/mL, demonstrate adherence and tolerance to HAART, and be absent of AIDS-defining illnesses. British and Italian groups exclude patients with prior or current infections that are considered to be high risk for reactivation with immune suppression (23,26). These infections include aspergillosis, other invasive fungal infections, active cytomegalovirus, recent influenza, recent RSV, recent severe bacterial infection, or incompletely treated mycobacterial infection. In contrast, the Spain and United States groups do not include all of these infections in their exclusion criteria (24,25). A more detailed list of inclusion and exclusion criteria is summarized in Table 1.
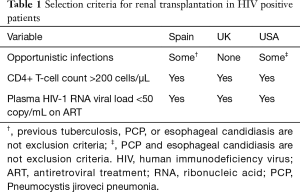
Full table
A substantial portion of HIV-positive patients also have concomitant hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. Additional screening for these diseases is recommended in HIV patients prior to transplantation because of an accelerated progression to end-stage liver disease and mortality after kidney transplantation in patients with HBV and HCV co-infections (27,28). As a result, patients with actively replicating HBV should be appropriately treated until they are aviremic to become candidates for transplantation. Special attention must be given to treat these patients with renally dosed medications and to continue their HAART (23). Besides medically optimizing and screening for common co-infections in HIV patients prior to transplantation, additional care must be given to the type of graft that is used. In general, live donation (related or unrelated donor) is preferable to cadaveric donation. British HIV association guidelines suggest that extended criteria donors are not used with deceased donors until there is more experience with transplantation in HIV-positive recipients (23). Lastly, live donors must also be informed that the recipient is HIV-positive and that the prognosis for both the graft and the patient may be worse than average.
Immunosuppressive protocols
Induction and maintenance
The first cases of kidney transplantation in HIV-positive patients showed greater than expected episodes of acute rejection (18). In general, the optimal immunosuppression regimen in these patients must balance the risks of worsening the underlying HIV infection and exposing the patient to opportunistic infections, while achieving adequate levels of immunosuppression to prevent graft rejection. Special care must also be given to maintain therapeutic and nontoxic levels of immunosuppressive agents, given the complicated pharmacokinetic interactions of these agents with some antiretroviral agents.
Maintenance immunosuppression in HIV-positive patients is not well established. Most centers use tacrolimus as a first line calcineurin inhibitor (CNI), however there are no studies that compare the effects of tacrolimus and cyclosporine on the progression of HIV infection. As previously mentioned, there are significant interactions between immunosuppressive agents and antiretroviral therapies (29). Patients treated with protease inhibitors (PIs) require substantially lower doses of CNI to achieve therapeutic blood levels (18,30). The immunosuppressive agent MMF may increase intracellular levels of abacavir, didanosine, and tenofovir, which may increase toxicity from these HIV medications (29). However, MMF has also been shown to have inhibitory effect on HIV replication in multiple studies (31-33). On a similar note, m-TOR inhibitors may provide an antiproliferative effect to help prevent solid organ transplantation-associated malignancies, such as Kaposi sarcoma (34). Overall, careful selection of immunosuppression and antiretroviral medications must be made to address patient-specific needs.
Attempts to improve the rates of acute rejection and graft survival were explored by using ATG induction. HIV-positive kidney transplantation patients who received ATG induction had a 61% lower risk of acute rejection at 1 year (aRR =0.39, 95% CI: 0.18–0.87, P=0.02) compared to those who did not receive induction therapy (35). However, ATG induction may severely deplete CD4 T cell counts and increase the risk of developing opportunistic infections. In fact, patients who received ATG induction therapy incurred twice as many serious infections and had an increased risk of death and graft loss compared to patients who did not receive induction therapy (14,18). Due to these risks and the narrow therapeutic window of ATG in this patient population, ATG induction therapy is only recommended for patients with a very high immunological risk for rejection (14,36). Belatacept-based immunosuppressive regimens may benefit HIV-positive kidney transplant patients. These regimens avoid drug interactions between CNI and antiretroviral medications, as well as decrease the risk and severity of non-immune toxicities, such as new-onset diabetes, hyperlipidemia and hypertension after transplantation (37,38). Lastly, polyclonal antibodies and OKT3 should not be used for induction or rescue therapy due to an increased risk of post-transplant lymphoproliferative disease, CMV, and other infections (39-41).
Allograft rejection and treatment
Acute rejection after kidney transplantation may be 2–3 folds higher in HIV-positive patients compared to HIV-negative patients (14); although, the etiology of this increased risk is not well understood. It has been suggested that patients with chronic HIV infection have an increased pathological immune activation (42-44). However, one study showed that patients with the highest levels of immune activation were less susceptible to rejection, suggesting that pathological immune activation is not the mechanism of increased allograft rejection in HIV patients (42). As previously mentioned, drug interactions between immunosuppressive and antiretroviral agents may have a role in the increased rejection rates. New antiretroviral medications that do not interact with CNIs, such as raltegravir, may offer more stable immunosuppressive regimens and lower the risk of acute rejection (45). The British HIV association recommends bolus therapy with high-dose methylprednisolone for initial episodes of acute rejection and graft nephrectomy after two or more episodes (23).
HIV therapy in kidney transplant recipients
Due to the complexities of these patients, an ideal ART regimen has not been established for HIV-positive kidney transplant recipients. As a result, general guidelines and recommendations for HIV-negative patients should be followed and adjusted to patient specific issues that arise (46,47). One suggested first line regimen is the combination of abacavir (or tenofovir as an alternative), lamivudine/emtricitabine, and raltegravir (48). Another study showed PI sparing ART regimens is superior than PI-containing in HIV-positive kidney transplant recipients (49). In general, the ideal ART regimen must maintain viral suppression and increased CD4 count, while preserving renal graft function and avoiding pharmacokinetic interactions with immunosuppressive medications.
Outcomes of kidney transplantation in HIV positive patients
The HIV infection progression and graft survival outcomes of HIV-positive kidney transplant patients have been largely positive. In one of the first and largest cohorts, 40 patients were reported to have 85% and 82% overall survival at 1 and 2 years, respectively (16). At 2 years, patients had undetectable plasma HIV-1 RNA levels, average CD4 counts of 4,400 cells/mm3, and no evidence of AIDS. However, acute rejection was noted to occur in 22% of patients (16). Preliminary results from a prospective cohort of 18 patients reported a 94% patient survival and an 83% graft survival at 3-year follow-up, which were consistent with the general transplant population (20). Plasma HIV RNA levels and CD4 counts remained stable, but acute graft rejection occurred in 52% and 70% of patients at 1 and 3 years, respectively (20). Despite the high incidences of graft rejection reported in these studies, a cohort of 8 patients noted 100% patient survival, 88% graft survival, and 13% acute rejection rates at 1 year follow-up (50). These small patient cohorts highlight the overall success of HIV infection progression and overall survival rates, but also underscore the high incidence of acute graft rejection in this patient population.
Perhaps the most generalizable results are from the largest prospective multicenter investigation that focused on the outcomes of kidney transplantation in HIV-positive patients. In this nonrandomized trial, 150 patients were followed for up to 3 years across 19 transplantation centers, without standardized immunosuppression regimens (14). Patient survival rates of 94.6% and graft survival rates of 90.4% were lower than those of HIV-negative patients, but comparable to rates of older HIV-negative transplant recipients. HIV infection progression was not seen in any of the patients. In a similar fashion as previously mentioned studies, the rejection rates of 31% and 41% at 1 and 3 years follow-up were 3–4 times as high as HIV-negative patients. The investigation also noted that patients who were treated for acute graft rejection were significantly more likely to incur graft loss (HR =2.8, 95% CI: 1.2–6.6, P=0.02) (14). While most studies report high acute graft rejection rates, a French group has achieved remarkably low rejection rates while adequately managing HIV infection (17). In a cohort of 27 HIV-positive kidney transplant recipients, patient survival was 100% and 98% at 1 and 2 years follow-up, respectively. Graft survival was 98% and 96% at 1 and 2 years follow-up, respectively. Although protease-inhibitor therapies were withdrawn from 11 patients due to high trough levels of CNI, HIV infection progression was not observed in any patients. Only 15% of patients experienced acute graft rejection, and one patient developed B-cell lymphoma. Patients were maintained on an immunosuppressive regimen that primarily included basiliximab for induction therapy, and a maintenance therapy of MMF, steroids, and tacrolimus or cyclosporine (17). Table 2 summarizes the outcomes of various HIV-positive kidney transplant studies. Harbell et al. reported the surgical complications in 275 in HIV-positive liver and/or kidney transplant recipients. He concluded the surgical outcomes are similar to what has been observed in the non-HIV setting in carefully selected HIV-infected liver and kidney transplant (51).

Full table
Future perspective
With early promising results from kidney transplantation in HIV-positive patients, we anticipate that future studies will include data with more than 3 years of follow-up. These studies should focus on lowering the high acute graft rejection rates to those of their HIV-negative counterparts. Additional topics to explore include the long-term survival of grafts and recipients, the long-term impact of immunosuppression on HIV viral loads and CD4 T cell counts, and the pharmacokinetic interactions between antiretroviral and immunosuppressive therapies. We believe that these questions will be answered with increasing experience with this patient population and properly designed prospective studies. The HIV Organ Policy Equity Act, or HOPE Act, the first large-scale clinical trial to study kidney transplantations between people with HIV has started at several clinical centers across the United States. The HOPE in Action Multicenter Kidney Study will determine the safety of this practice by evaluating kidney recipients for potential transplant-related and HIV-related complications following surgery. The study is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
Conclusions
In summary, kidney transplantation can be a safe and effective form of renal replacement therapy in carefully selected HIV-positive patients. Patients with HIV infection well-controlled with ART prior to transplantation who are negative for HBV and HCV infections are ideal candidates. Patients should continue a stable ART after transplantation. Future studies should focus on improving acute graft rejection rates and understanding antiretroviral and immunosuppression medication interactions. Early results suggest that kidney transplantation in HIV-positive patients will continue to be a viable treatment for ESRD in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rao TK, Filippone EJ, Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 1984;310:669-73. [Crossref] [PubMed]
- Pardo V, Aldana M, Colton RM, et al. Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med 1984;101:429-34. [Crossref] [PubMed]
- Gardenswartz MH, Lerner CW, Seligson GR, et al. Renal disease in patients with AIDS: a clinicopathologic study. Clin Nephrol 1984;21:197-204. [PubMed]
- Shahinian V, Rajaraman S, Borucki M, et al. Prevalence of HIV-associated nephropathy in autopsies of HIV-infected patients. Am J Kidney Dis 2000;35:884-8. [Crossref] [PubMed]
- Ahuja TS, Borucki M, Funtanilla M, et al. Is the prevalence of HIV-associated nephropathy decreasing? Am J Nephrol 1999;19:655-9. [Crossref] [PubMed]
- Winston JA, Bruggeman LA, Ross MD, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med 2001;344:1979-84. [Crossref] [PubMed]
- Roling J, Schmid H, Fischereder M, et al. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis 2006;42:1488-95. [Crossref] [PubMed]
- Daugas E, Rougier JP, Hill G. HAART-related nephropathies in HIV-infected patients. Kidney Int 2005;67:393-403. [Crossref] [PubMed]
- Cohen MS, Shaw GM, McMichael AJ, et al. Acute HIV-1 Infection. N Engl J Med 2011;364:1943-54. [Crossref] [PubMed]
- Rodriguez RA, Mendelson M, O'Hare AM, et al. Determinants of survival among HIV-infected chronic dialysis patients. J Am Soc Nephrol 2003;14:1307-13. [Crossref] [PubMed]
- Spital A. Should all human immunodeficiency virus-infected patients with end-stage renal disease be excluded from transplantation? The views of U.S. transplant centers. Transplantation 1998;65:1187-91. [Crossref] [PubMed]
- European Best Practice Guidelines for Renal Transplantation (part 1). Nephrol Dial Transplant 2000;15 Suppl 7:1-85. [PubMed]
- Coffman KL. Evidence-based medicine: the dilemma of transplantation in patients with HIV infection. Curr Opin Organ Transplant 2004;9:422-7. [Crossref]
- Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med 2010;363:2004-14. [Crossref] [PubMed]
- Pelletier SJ, Norman SP, Christensen LL, et al. Review of transplantation in HIV patients during the HAART era. Clin Transpl 2004.63-82. [PubMed]
- Kumar MS, Sierka DR, Damask AM, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int 2005;67:1622-9. [Crossref] [PubMed]
- Touzot M, Pillebout E, Matignon M, et al. Renal transplantation in HIV-infected patients: the Paris experience. Am J Transplant 2010;10:2263-9. [Crossref] [PubMed]
- Stock PG, Roland ME, Carlson L, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: a pilot safety and efficacy study. Transplantation 2003;76:370-5. [Crossref] [PubMed]
- Abbott KC, Swanson SJ, Agodoa LY, et al. Human immunodeficiency virus infection and kidney transplantation in the era of highly active antiretroviral therapy and modern immunosuppression. J Am Soc Nephrol 2004;15:1633-9. [Crossref] [PubMed]
- Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant 2008;8:355-65. [Crossref] [PubMed]
- Tan HP, Kaczorowski DJ, Basu A, et al. Living-related donor renal transplantation in HIV+ recipients using alemtuzumab preconditioning and steroid-free tacrolimus monotherapy: a single center preliminary experience. Transplantation 2004;78:1683-8. [Crossref] [PubMed]
- EBPG (European Expert Group on Renal Transplantation). European Renal Association (ERA-EDTA). European Society for Organ Transplantation (ESOT). European Best Practice Guidelines for Renal Transplantation (part 1). Nephrol Dial Transplant 2000;15 Suppl 7:1-85.
- Bhagani S, Sweny P, Brook G. Guidelines for kidney transplantation in patients with HIV disease. HIV Med 2006;7:133-9. [Crossref] [PubMed]
- Solid organ transplantation in the HIV-infected patient. Am J Transplant 2004;4 Suppl 10:83-8. [Crossref] [PubMed]
- Miro JM, Torre-Cisnero J, Moreno A, et al. GESIDA/GESITRA-SEIMC, PNS and ONT consensus document on solid organ transplant (SOT) in HIV-infected patients in Spain (March, 2005). Enferm Infecc Microbiol Clin 2005;23:353-62. [PubMed]
- Grossi PA, Tumietto F, Costigliola P, et al. Liver transplantation in HIV-infected individuals: results of the Italian national program. Transplantation 2006;82:446. [Crossref]
- Pereira BJ. Renal transplantation in patients positive for hepatitis B or C (con). Transplant Proc 1998;30:2070-2. [Crossref] [PubMed]
- Parfrey PS, Forbes RD, Hutchinson TA, et al. The clinical and pathological course of hepatitis B liver disease in renal transplant recipients. Transplantation 1984;37:461-6. [Crossref] [PubMed]
- Izzedine H, Launay-Vacher V, Baumelou A, et al. Antiretroviral and immunosuppressive drug-drug interactions: an update. Kidney Int 2004;66:532-41. [Crossref] [PubMed]
- Jain AK, Venkataramanan R, Shapiro R, et al. The interaction between antiretroviral agents and tacrolimus in liver and kidney transplant patients. Liver Transpl 2002;8:841-5. [Crossref] [PubMed]
- Chapuis AG, Paolo Rizzardi G, D'Agostino C, et al. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med 2000;6:762-8. [Crossref] [PubMed]
- Hossain MM, Coull JJ, Drusano GL, et al. Dose proportional inhibition of HIV-1 replication by mycophenolic acid and synergistic inhibition in combination with abacavir, didanosine, and tenofovir. Antiviral Res 2002;55:41-52. [Crossref] [PubMed]
- Sankatsing SU, Hoggard PG, Huitema AD, et al. Effect of mycophenolate mofetil on the pharmacokinetics of antiretroviral drugs and on intracellular nucleoside triphosphate pools. Clin Pharmacokinet 2004;43:823-32. [Crossref] [PubMed]
- Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 2005;352:1317-23. [Crossref] [PubMed]
- Locke JE, James NT, Mannon RB, et al. Immunosuppression regimen and the risk of acute rejection in HIV-infected kidney transplant recipients. Transplantation 2014;97:446-50. [Crossref] [PubMed]
- Suarez JF, Rosa R, Lorio MA, et al. Pretransplant CD4 Count Influences Immune Reconstitution and Risk of Infectious Complications in Human Immunodeficiency Virus-Infected Kidney Allograft Recipients. Am J Transplant 2016;16:2463-72. [Crossref] [PubMed]
- Ebcioglu Z, Liu C, Shapiro R, et al. Belatacept Conversion in an HIV-Positive Kidney Transplant Recipient With Prolonged Delayed Graft Function. Am J Transplant 2016;16:3278-81. [Crossref] [PubMed]
- Cohen EA, Mulligan D, Kulkarni S, et al. De Novo Belatacept in a Human Immunodeficiency Virus-Positive Kidney Transplant Recipient. Am J Transplant 2016;16:2753-7. [Crossref] [PubMed]
- Portela D, Patel R, Larson-Keller JJ, et al. OKT3 treatment for allograft rejection is a risk factor for cytomegalovirus disease in liver transplantation. J Infect Dis 1995;171:1014-8. [Crossref] [PubMed]
- Boland GJ, Hene RJ, Ververs C, et al. Factors influencing the occurrence of active cytomegalovirus (CMV) infections after organ transplantation. Clin Exp Immunol 1993;94:306-12. [Crossref] [PubMed]
- Hesse UJ, Wienand P, Baldamus C, et al. The risk of infection following OKT3 and antilymphocyte globulin treatment for renal transplant rejection: results of a single center prospectively randomized trial. Transpl Int 1992;5 Suppl 1:S440-3. [Crossref] [PubMed]
- Lorio MA, Rosa R, Suarez JF, et al. Influence of immune activation on the risk of allograft rejection in human immunodeficiency virus-infected kidney transplant recipients. Transpl Immunol 2016;38:40-3. [Crossref] [PubMed]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365-71. [Crossref] [PubMed]
- Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015;175:88-99. [Crossref] [PubMed]
- Tricot L, Teicher E, Peytavin G, et al. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant 2009;9:1946-52. [Crossref] [PubMed]
- Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. Jama 2010;304:321-33. [Crossref] [PubMed]
- [AIDS Study Group/Spanish AIDS Plan consensus document on antiretroviral therapy in adults with human immunodeficiency virus infection (updated January 2010)]. Enferm Infecc Microbiol Clin 2010;28:362.e1-91.
- Trullas JC, Cofan F, Tuset M, et al. Renal transplantation in HIV-infected patients: 2010 update. Kidney Int 2011;79:825-42. [Crossref] [PubMed]
- Rosa R, Suarez JF, Lorio MA, et al. Impact of antiretroviral therapy on clinical outcomes in HIV (+) kidney transplant recipients: Review of 58 cases. F1000Res 2016;5:2893. [Crossref] [PubMed]
- Gruber SA, Doshi MD, Cincotta E, et al. Preliminary experience with renal transplantation in HIV+ recipients: low acute rejection and infection rates. Transplantation 2008;86:269-74. [Crossref] [PubMed]
- Harbell J, Fung J, Nissen N, et al. Surgical complications in 275 HIV-infected liver and/or kidney transplantation recipients. Surgery 2012;152:376-81. [Crossref] [PubMed]


