
Transurethral resection of bladder tumours: established and new methods of tumour visualisation
Introduction
Transurethral resection (TUR) of bladder tumours ensures histological diagnosis including information on the completeness of resection, histological variant as well as grading and invasiveness of the tumour (1).
When performing TUR, one must circumvent several obstacles to achieve complete resection of the tumour. First, TUR training seems to be subject to a learning curve (2). This learning curve does not solitarily consist of technical aspects of surgery but also comprises the visual differentiation between healthy and suspicious tissue. Regular white light cystoscopy (WLC) bears the risk of missing mainly flat lesions (3). As a result, vital tumour could remain undetected as well as untreated and might eventually progress.
During recent years, technical innovations improved the intraoperative detection and visibility during TUR. The most important techniques, which have individually found their way into international guidelines, are photodynamic imaging (PDI) and narrowband imaging (NBI) (1). Furthermore, there are more or less experimental approaches such as optical coherence tomography (OCT), confocal laser endomicroscopy (CLE), red/green/blue analysis (RGB) of WLC, and the use of artificial intelligence (4-7). Moreover, the combination of two or more techniques in a multiparametric setting describes another development in improving intraoperative imaging (8).
All of these new developments need to demonstrate their additional value by improving tumour detection, recurrence rate, and overall prognosis in comparison to regular WLC.
The aim of this review is to describe today’s knowledge of the more established methods for tumour visualization and to depict the most recent developments in intraoperative imaging.
Methods and material
This review article aims to summarize the current published methods of bladder tumour visualization while performing transurethral resection. A nonsystematic review of relevant PubMed literature was performed. Search items were “photodynamic imaging”, “narrowband imaging”, “optical coherence tomography”, and “confocal laser endomicroscopy”, each combined with the search item “bladder cancer”.
Narrow band imaging
NBI narrows the bandwith of the emitted light to achieve different depths of penetration (9). Only the wavelengths 415 and 540 nm permeate, which results in an enhanced contrast between the superficial mucosa and microvascular structures (10) (Figure 1).
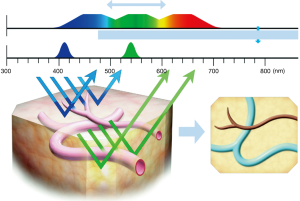
Specialized cystoscopes are required to apply NBI. However, in contrast to PDI, there is no need for instillation of an exogenous fluorescent compound. First, a regular WLC is conducted. Afterwards, NBI is activated in order to find additional tumours and/or to enhance identification of suspicious lesions (Figure 2). If a suspicious area or macroscopic tumour is found, TUR or cold-cup biopsies will be performed. A meta-analysis by Xiong et al. showed that NBI increases tumour detection when compared to WLC with a pooled additional detection rate (ADR) of 9.9% (11). Bryan et al. showed similar results comparing untrained users of NBI cystoscopy with trained endourologists. In this short series consisting of 52 patients, WLC detected an overall of 108 tumours, 64 of whom were detected in 29 patients by a trained urologist, while 44 tumours were detected in 23 patients by a first time user. After switching to NBI, the experienced surgeon, as well as the new user, detected additional 15 tumours, resulting in 138 detected tumours in combination. These additional urothelial carcinomas were flat lesions in 40% of the cases. Wilcoxon (unpaired) signed rank test (P=0.74) showed no statistical difference between the ADR of the trained urologist and the trainee respectively. These results did not only suggest a benefit regarding the detection rate. Moreover, they demonstrate that surgeons might benefit from this technique not only in their learning curve but also as a TUR expert. Therefore, NBI might not merely be a training tool to improve a urologist’s WLC skills during the learning curve but also may have its place in daily routine (12).
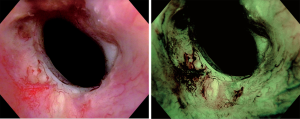
Compared to WLC, there is a significant influence on recurrence-free survival (RFS). Another meta-analysis by Kang et al. showed a pooled recurrence rate (RR) after NBI guided TUR at month 3 and 12 months of 4.6% and 26.0%, respectively. In contrast, patients that underwent WLC guided TUR had a pooled recurrence rate in the same time intervals of 16.7% and 38.6%, respectively (13). A prospective, randomized single blinded multicenter study conducted by the Clinical Research Office of the Endourological Society (CROES) by Naito et al. corroborate a significantly higher RR at 12 months when comparing WLC to WLC+NBI (WLC: 27.3% vs. WLC + NBI: 5.6%; P=0.002) (14).
Furthermore, Geavlete and colleagues additionally showed that the specificity for identifying a carcinoma in situ (CIS) was significantly higher with NBI when compared to WLC (53.8% versus 15.4%). On the downside, the rate of false positive biopsies, for Ta as well as T1 tumours (pTa: 14.3% vs. 9.6% and pT1: 8.1% vs. 5.1% respectively), was elevated by the use of NBI (15). This suggests a potential risk of overtreatment by the use of NBI.
Taken together, NBI represents an easily applicable technique, which improves ADR as well as the rate of recurrence. It does not require preparations in advance and trainees can be taught easily and benefit from the advantages of NBI from the start.
Photodynamic imaging
As with NBI, PDI requires specialized cystoscopes but in addition a blue light source to perform TUR (16). Before PDI-TUR, a photodynamic compound has to be instilled intravesically one to four hours prior surgery. Up to now, two substances have been proven to act as a photosensibilisator. These substances are 5-aminolevulinic acid (ALA) and its derivative hexaminolevulinate (HAL), with only the latter being approved for clinical use. Both substances accumulate within the cells of the tumour and are metabolized to protoporphyrin IX, which leads to a red fluorescence of the tumour under blue light (Figures 3,4).
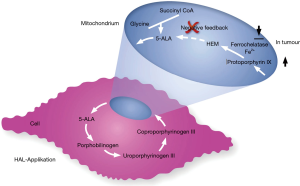
Gallagher examined 345 patients, who had received either a “good quality” WLC-TUR (n=153) or a “good quality” PDI-TUR (n=192). Time to recurrence with PDI was significantly longer than with WLC (PDD: 52.9 vs. WLC: 42.4 months, P=0.001). The RR reflects the aforementioned result after one year (WLC: 38.9% vs. PDI: 21.5%, P value <0.001) and three years, respectively (WLC: 53.3% vs. PDI: 39.0%, P=0.02). Looking at the EAU 2002 risk groups individually, these results were consistent. Furthermore, binary logistical and Cox regression analyses suggested that this benefit was independent of the surgeon (17). These results were confirmed by Lykke et al. who presented similar results with a reduction of RR by the use of PDI by 41% compared to regular WLC (18).
As the detection of flat lesions such as CIS can be challenging, the ADR of CIS reported in a retrospective cohort of 70 patients is especially interesting. The study population underwent WLC-TUR as well as PDI-TUR between 2009 and 2011. WLC revealed an overall of 46 tumours ranging from CIS to T2. Using PDI revealed 29 additional CIS leading to an overall detection of 79 lesions. Interestingly, WLC did not detect any tumour in 11 of these cases. This results in an ADR of 36.7% for CIS alone (3).
As mentioned above, PDI requires prior instillation of HAL. This leads to additional urethral manipulation before the actual operation and might cause patients’ discomfort. A possible solution to this dilemma could be the oral administration of ALA three to four hours before TUR. In 2016, Kata et al. retrospectively analyzed 69 patients with suspected panurothelial disease, who underwent cystoscopy as wells as upper urinary tract endoscopy, for which oral ALA was originally intended (19). These patients had been given 20-mg/kg body weight prior to surgery. Oral ALA followed by PDI lead to the detection of 16 more lesions as compared to WLC resulting in an ADR of 23.1% while avoiding discomfort caused by additional catheterization. The rate of false positive biopsies with oral ALA followed by PDI was higher when compared to WLC (35.5% vs. 28.5%) (19). In 2013, O’Brien and colleagues postulated an average false positive rate of 15–20% for the regular intravesical use of HAL, while randomized controlled trials (RCT) to compare oral and intravesical photosensibilisators are still missing (20).
Lykke et al. [2015] also portrayed the financial impact of using PDI. The utilization of PDI in connection with postoperative Mitomycin C, whenever reasonable, resulted in additional costs of 933€ (1.091$) per patient. As RFS was prolonged in the PDI group, savings of 1.135€ (1.327$) during the first year of follow-up were estimated according to the conditions of the Danish medical system (18).
The meta-analysis of 15 RCTs by Lee et al. [2015] compared ALA-PDI, HAL-PDI, NBI, and WLC. Network meta-analysis showed that ALA-PDI, HAL-PDI, and NBI were all superior to WLC with regard to RR. The analysis also showed that ALA-PDI was superior to both HAL-PDI and NBI (HAL: OR =0.48, 95% CI: 0.26–0.95; NBI: OR =0.53, 95% CI: 0.26–1.09). Comparing NBI and ALA-PDI, on the other hand, showed no significant difference in RR (OR =1.11, 95% CI: 0.55–2.1). No technique was superior in terms of improving the rate of progressive disease (21). However, only HAL has been available for performing PDI in clinical routine.
Optical coherence tomography
While PDI and NBI both represent an improvement on a macroscopic level, OCT represents a technical innovation on a microscopic scale (7). Comparable to everyday ultrasound, OCT produces a real-time image of the examined tissue. Unlike ultrasound, OCT does not rely on sonic waves, but measures changes of backscattered light emitted by a Michelson interferometer instead. Those changes are caused by asymmetric structures within the tissue. The technique enables the surgeon to produce an image of the microstructure with a penetration depth of up to 2 mm (22,23). OCT has found widespread use throughout many medical fields, such as ophthalmology, gynecology, gastroenterology and cardiology. In 2015, Kiseleva examined this technique and its ability to differentiate between mucosal pathologies of the human bladder. Cystoscopy was performed in n=68 patients with chronic cystitis or suspicion of bladder cancer. Five additional volunteers were examined to act as the control. As OCT only covers small areas, the focus of this technique does not lie within the detection of additional lesions, but rather in improving the distinction between healthy and suspicious tissue.
Cystoscopy of those 73 patients resulted in 96 pictures, which were compared to the histopathological findings of TUR-biopsies taken from the same spot. The pathological results and OCT findings were each classified into seven groups:
“1. Normal”, “2. Severe fibrosis at chronic inflammation”, “3. Scar”, “4. Acute inflammation”, “5. Carcinoma in situ”, “6. Flat transitional cell carcinoma, stage I-IIa”, and “7. Recurrence of carcinoma in scar”. An overall of 36 carcinomas was found (groups 5–7). Afterwards, pathological and OCT findings were compared. The collation of groups 5+6 and group 4 resulted in a sensitivity of 86%, a specificity of 68%, and a diagnostic accuracy (DA) of 75%. Comparison of groups 7 and 3 brought forth a sensitivity of 93%, a specificity of 99%, and a DA of 97% (24). Huang et al. performed an extensive meta-analysis with regards to the DA of OCT. The sensitivity and specificity of the analyzed publications ranged from 76% to 100% and 62% to 97%, resulting in a pooled sensitivity and specificity of 0.96 (95% CI: 0.94–0.98) and 0.82 (95% CI: 0.80–0.85), respectively. DA was assessed by using summary receiver operating characteristics (sROC) and the Q* index. This resulted in a DA of 97.35% and 92.57%, respectively (22).
However, the possible existence of a learning curve or the user-friendliness of the device have not been examined yet rendering it difficult to evaluate the adaptability of this technique in an everyday clinical setting.
Confocal laser endomicroscopy
As for OCT, CLE had been used for in other medical areas before finding its way to the urological field. Both techniques bear several similarities, as they both rely on optical probes and only have a limited examination field. Unlike OCT, which produces an image reminiscent of an ultrasound picture, CLE provides the surgeon with a black and white picture resembling a histological slice. In order to provide such a picture, CLE requires a preoperative application of fluorescein. Following either an intravesical instillation or an intravenous administration of the drug, a 488 nm low-power laser scans the tissue and excites the fluorescein. The emitted in-focus light is measured by a photodetector, resulting in the well-nigh histological image (4) (Figure 5).
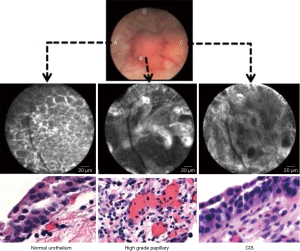
Due to the availability of different probe diameters, CLE can be used to examine urothelial tissue beyond the bladder in the upper urinary tract.
So far, large-scale studies examining the DA of CLE, as well as its specificity and sensitivity, are missing. On a small scale, Chang examined the interobserver agreement (IA) of CLE. For this purpose, 17 trained as well as untrained urologists, pathologists, and non-clinical researchers underwent a video-based training. Afterwards, those examiners evaluated a new set of CLE, WLC, and CLE + WLC sequences. Experienced urologists reached an IA of 90% on CLE and WLC + CLE, but only 74% only WLC alone. Untrained urologists, on the other hand, obtained an IA of 77% on CLE, 78% on WLC and 80% on WLC + CLE. Non-clinical researchers and pathologists reached an IA on CLE alone of 77% and 81%, respectively. Sensitivity ranged from 75% to 89%, while specificity ranged from 73% to 88%. Maximum values were spread across all examined groups (25). This data suggests a learning curve to expertly perform CLE supported cystoscopy and TUR.
Red/green/blue analysis
All the above techniques require special equipment such as light sources, filters, cystoscopes, or probes. In contrast, Hah et al. performed a double-blinded prospective study investigating the connection between numerical information of red/green/blue (RGB) values of WLC or NBI and the detection of bladder cancer (6). Another study of the same research group examined the ability of RGB analysis to detect CIS and to predict bacillus Calmette-Guérin (BCG) failure (26). WLC pictures of suspicious lesions and healthy tissue were parsed using a standard picture archiving and communication system (PACS). Three randomized areas, sized 1×1 Pixel, from each TUR-site were analyzed for their respective RGB values. Both studies suggested that the R-value from WLC was a significant predictor for the detection of bladder cancer. They also showed that using the R-value also helped in differentiating between CIS, acute cystitis and interstitial cystitis. A WLC R-value ≥175 (OR =3.28, P<0.001) was also a predictive factor for BCa recurrence.
WLC B-values >73 and WLC G-values >97 were also a significant predictor of CIS in abnormal lesions (B-value: OR =2.94, P=0.008; G-value: OR =0.46, P<0.001). Pairwise comparison of receiver operating characteristic curves showed that WLC B-value, NBI B-value, and NBI G-value were predictors of muscle invasion, while WLC R-value and NBI G-value were predictors of high-grade BCa.
The development of RGB analysis represents a new building block in improving BCa diagnosis. As the readily available PACS is used to determine the color values, RGB analysis provides a cost-effective tool, which, so far, cannot be performed immediately. With the further improvement and implementation of this principle, future real-time RGB analysis might provide the missing link between WLC and high technology devices.
Artificial intelligence
In many medical and non-medical fields, Artificial Intelligence (AI) finds increasing practical use. Sofar most developments have been made by creating algorithms analyzing radiological images such as CT or MRI scans (27). The methods applied range from deep convolutional neural networks (DCNN) to variation autoencoders (28). The training process involved is the same for almost all technologies:
Pictures and scans, which were already interpreted by a human professional, were shown to the system together with the results. Subsequently, the system is confronted with a set of evaluation samples. In 2018, Ikeda reported on training a DCNN with 143 pictures of histologically confirmed tumours of the bladder and 107 pictures of the regular bladder mucosa. The pictures were analyzed using the Inception-V3 code developed for ImageNet. This neuronal network was pre-trained using 1.2 million entire images, which were then classified in 1,000 categories. Afterwards, the system was tested with pictures of 34 tumourous and 28 normal areas. The test revealed a sensitivity of 93.1% and a specificity of 95.5% for the systems ability to differentiate between healthy tissue and bladder tumours. This results in a Youden’s index of 0.886, representing a high capability to distinguish between the two subsets. Furthermore, the results depict a DA of 94.1% (5).
Up to now, there is no real-time application of AI at cystoscopic images. Like RGB analysis, further research and interface-development are needed to provide an additional WLC-based tool to detect BCa.
Combination of different methods for tumour visualization
As already mentioned above, the use of only one technique bears the disadvantage of missing another technique’s benefits. Combining two or more modalities could lead to an improved tumour detection. Until now, the simultaneous application of several techniques is limited by the technical requirements to the cystoscope, the light filters, and the light detection. Some of the above mentioned techniques such as PDI or NBI improve the detection rate at the cost of a higher false positive rate, resulting in additional unnecessary TUR biopsies. Combining these techniques with OCT or CLE which are not suitable for a complete scan of the bladder and thus the detection of additional lesions could result in a higher ADR a lower additional false positive biopsies.
Using OCT and CLE, surgeons might be able to identify borders of suspicious lesions more easily. This might lead to a more precise and complete TUR and thus to a higher RFS and, possibly, a higher PFS. Further large-scale studies on this hypothesis are yet to be done.
Kriegmair et al. [2018] addressed this problem by developing the appropriate hardware as well as the corresponding software. For this purpose, a hybrid cystoscope, equipped with a sCMOS camera and a 30° optic, was fitted with a LED light source capable of different spectra. This outfit enables the surgeon to project the same picture section with WLC, PDI NBI-like and autofluorescence imaging (AF). Combining those techniques results in 6 synchronous projections of the same picture with a real multiparametric merge. After completion of rigorous ex vivo and subsequent in vivo testing in animals, this multiparametric approach was tested in overall 12 humans. During these first tests of the prototype, multiparametric images were created successfully with additional lesions detected by fusing PDI, NBI-like, and AF (8).
This multiparametric approach probably represents the most pioneering concept. By developing the hardware and software to successfully produce and fuse multiparametric images, the gap between WLC and other techniques at hand might be bridged. However, it is yet to be tested in a TUR setting.
Conclusions
Transurethral resection constitutes the most important factor in the diagnosis of BCa and the therapy of NMIBC. As the presented studies show, WLC suffers the inherent disadvantage of missing 10–37% of all present tumours. The technique’s weak point mainly lies in the detection of flat lesions or CIS. Advances in medical technology have brought forth different innovations to tackle these shortcomings.
NBI, as well as PDI, have been shown to address this issue and to provide an additional benefit by improving a tumour’s detectability for the surgeon on the one hand and to significantly reduce the RR on the other hand. While both techniques have certain disadvantages such as the need for preoperative instillations or the elevated rate of false positive biopsies, they both have found their way into the EAU’s guidelines for the diagnosis of BCa (1).
CLE and OCT have not yet made this decisive step, even though they have been shown to address the weaknesses of NBI and PDI by potentially improving the rate of false positive biopsies. Leading to the conclusion that combining two or more techniques has to be the next inevitable step. One of the most promising ventures in recent time is the real-time multiparametric fusion presented by Kriegmair (8). Other concepts, such as the use of AI or RGB analysis, are interesting concepts but need further developing and research to add a significant benefit to the transurethral resection of bladder tumours.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Roghmann reports personal fees from Ipsen.
References
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Kruck S, Bedke J, Hennenlotter J, et al. Virtual bladder tumor transurethral resection: an objective evaluation tool to overcome learning curves with and without photodynamic diagnostics. Urol Int 2011;87:138-42. [Crossref] [PubMed]
- Sfetsas K, Mitropoulos D. Reducing understaging of bladder cancer with the aid of photodynamic cystoscopy. J Egypt Natl Canc Inst 2016;28:89-94. [Crossref] [PubMed]
- Chen SP, Liao JC. Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: a new era of optical diagnosis? Curr Urol Rep 2014;15:437. [Crossref] [PubMed]
- Ikeda A, Hoshino Y, Nosato H, et al. Objective evaluation for the cystoscopic diagnosis of bladder cancer using artificial intelligence. 2018 EAU Annual Congress; 03-19-2018; Copenhagen 2018.
- Hah YS, Lee KS, Koo KC, et al. Identification of red/green/blue values from white-light imaging and narrow-band imaging for the discrimination of bladder cancer features. 2018 EAU Annual Congress; 03-19-2018; Copenhagen 2018.
- Tearney GJ, Brezinski ME, Southern JF, et al. Optical biopsy in human urologic tissue using optical coherence tomography. J Urol 1997;157:1915-9. [Crossref] [PubMed]
- Kriegmair MC, Theuring M, Rother J, et al. Multiparametric cystoscopy for the detection of bladder cancer using wide field multispectral imaging during TUR-B. 2018 EAU Annual Congress; Copenhagen 2018.
- Bryan RT, Billingham LJ, Wallace DM. Narrow-band imaging flexible cystoscopy in the detection of recurrent urothelial cancer of the bladder. BJU Int 2008;101:702-5; discussion 705-6. [Crossref] [PubMed]
- Aeishen S, Dawood Y, Papadoukakis S, et al. Supplementary optical techniques for the detection of nonmuscle invasive bladder cancer. Urologe A 2018;57:139-47. [Crossref] [PubMed]
- Xiong Y, Li J, Ma S, et al. A meta-analysis of narrow band imaging for the diagnosis and therapeutic outcome of non-muscle invasive bladder cancer. PLoS One 2017;12:e0170819. [Crossref] [PubMed]
- Bryan RT, Shah ZH, Collins SI, et al. Narrow-band imaging flexible cystoscopy: a new user's experience. J Endourol 2010;24:1339-43. [Crossref] [PubMed]
- Kang W, Cui Z, Chen Q, et al. Narrow band imaging-assisted transurethral resection reduces the recurrence risk of non-muscle invasive bladder cancer: A systematic review and meta-analysis. Oncotarget 2017;8:23880-90. [PubMed]
- Naito S, Algaba F, Babjuk M, et al. The Clinical Research Office of the Endourological Society (CROES) Multicentre Randomised Trial of Narrow Band Imaging-Assisted Transurethral Resection of Bladder Tumour (TURBT) Versus Conventional White Light Imaging-Assisted TURBT in Primary Non-Muscle-invasive Bladder Cancer Patients: Trial Protocol and 1-year Results. Eur Urol 2016;70:506-15. [Crossref] [PubMed]
- Geavlete BF, Brinzea A. Carcinoma in situ of the urinary bladder - from pathology to narrow band imaging. Rom J Morphol Embryol 2015;56:1069-76. [PubMed]
- Olympus. Olympus blue-light manual. Olympus Europa SE & Co. KG.
- Gallagher KM, Gray K, Anderson CH, et al. 'Real-life experience': recurrence rate at 3 years with Hexvix((R)) photodynamic diagnosis-assisted TURBT compared with good quality white light TURBT in new NMIBC-a prospective controlled study. World J Urol 2017;35:1871-7. [Crossref] [PubMed]
- Lykke MR, Nielsen TK, Ebbensgaard NA, et al. Reducing recurrence in non-muscle-invasive bladder cancer using photodynamic diagnosis and immediate post-transurethral resection of the bladder chemoprophylaxis. Scand J Urol 2015;49:230-6. [Crossref] [PubMed]
- Kata SG, Zreik A, Ahmad S, et al. Concurrent bladder cancer in patients undergoing photodynamic diagnostic ureterorenoscopy: how many lesions do we miss under white light cystoscopy? Cent European J Urol 2016;69:334-40. [PubMed]
- O'Brien T, Ray E, Chatterton K, et al. Prospective randomized trial of hexylaminolevulinate photodynamic-assisted transurethral resection of bladder tumour (TURBT) plus single-shot intravesical mitomycin C vs conventional white-light TURBT plus mitomycin C in newly presenting non-muscle-invasive bladder cancer. BJU Int 2013;112:1096-104. [Crossref] [PubMed]
- Lee JY, Cho KS, Kang DH, et al. A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer 2015;15:566. [Crossref] [PubMed]
- Huang J, Ma X, Zhang L, et al. Diagnostic accuracy of optical coherence tomography in bladder cancer patients: A systematic review and meta-analysis. Mol Clin Oncol 2018;8:609-12. [PubMed]
- Wang HW, Chen Y. Clinical applications of optical coherence tomography in urology. Intravital 2014;3:e28770. [Crossref] [PubMed]
- Kiseleva E, Kirillin M, Feldchtein F, et al. Differential diagnosis of human bladder mucosa pathologies in vivo with cross-polarization optical coherence tomography. Biomed Opt Express 2015;6:1464-76. [Crossref] [PubMed]
- Chang TC, Liu JJ, Hsiao ST, et al. Interobserver agreement of confocal laser endomicroscopy for bladder cancer. J Endourol 2013;27:598-603. [Crossref] [PubMed]
- Hah YS, Lee KS, Koo KC, et al. Identifying carcinoma in situ lesions in the bladder using red/green/blue numerical values from white-light imaging. 2018 EAU Annual Congress 03-19-2018; Copenhagen 2018.
- Thompson RF, Valdes G, Fuller CD, et al. Artificial intelligence in radiation oncology: A specialty-wide disruptive transformation? Radiother Oncol 2018;129:421-6. [Crossref] [PubMed]
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer 2018;18:500-10. [Crossref] [PubMed]


