
Prediction tools in non-muscle invasive bladder cancer
Introduction
Bladder cancer (BCa) is the second most common genitourinary malignancy with 81,190 estimated new diagnosis in 2018 in the United States only (1). Approximately 75–85% of all patients with BCa present at diagnosis a non-muscle invasive BCa (2). Therefore, this condition is very heterogeneous, and it is burdened by variable but high rates of short- and long-term recurrence and progression. Given the availability of several therapeutic options such as surveillance after resection, single instillations, intravesical therapy with chemo- or immunotherapeutic agents and early cystectomy, a risk-based therapeutic approach should be always applied by urologists to propose the most efficient treatment for each case. Moreover, despite the clinical similar characteristics of diseases, the response to intravesical chemotherapy is variable and the follow-up should be always targeted on the risk of recurrence and progression.
Finding clinical, pathological and molecular features seems to be more and more necessary for stratifying patients in groups with different prognosis, and consequently to help urologist in the decision making of non-muscle invasive BCa. This review highlights the current available tools to predict risk of recurrence and progression in non-muscle invasive BCa.
Evidence acquisition
We searched the Medline/PubMed databases for the comprehensive information on the prediction tools in non-muscle invasive BCa. The terms used for the search included “bladder cancer”, “non-muscle invasive”, “NMIBC”, “BCa”, “tools”, “prediction”, “predictors”, “recurrence”, “progression”, “clinical”, “molecular”, “biochemical”.
Clinical predictors
European Organization for Research and Treatment of Cancer (EORTC) and Club Urologico Espanol de Tratamiento Oncologico (CUETO) risk tables
In 2006, EORTC proposed risk assessment tables to predict short- and long-term recurrence and progression in non-muscle invasive BCa and currently this is the most frequently applied and validated model (3). Data of 2,596 patients with Ta and T1 neoplasms, included in seven EORTC randomized studies between January 1979 and September 1989, were analyzed. This tables are founded on clinical/pathologic characteristics which showed the strongest association with outcomes. These variables included tumor stage, grade [World Health Organization (WHO) 1973] (4), number of tumors, size of tumors, presence of concomitant carcinoma in situ (CIS) and previous disease recurrence. A multivariable analysis was built to assess a weight score for each factor. The recurrence risk-table includes a score from 0 to 17 whereas the progression score is from 0 to 23, and patients were divided in four prognostic groups. However, 21% of the patients included in these studies have not been treated with intravesical therapy and patients who received Bacillus Calmette Guerin (BCG, 7%) have been treated with induction course but not with maintenance.
On the contrary, the Club Urologico Espanol de Tratamiento Oncologico (CUETO) proposed risk-assessment tables to predict short- and long-term risk of recurrence and progression specifically for patients treated with BCG. The results were based on analyses on 1,062 patients who received BCG instillations: the scoring system was calculated with a score from 0 to 16 and from 0 to 14 for recurrence and progression, respectively. It was based of seven variables which include age, gender, prior recurrence, number of tumors, tumor stage and grade, and presence of concomitant CIS. Patients were classified into four groups by score.
External validations of EORTC and CUETO risk tables
Several studies have been performed to externally validate these two scoring systems. Xylinas et al. (5) evaluated their efficacy on 4,689 patients, founding that both risk tables overestimate disease recurrence and progression. Same result was reported by Fernandez-Gomez et al. (6), who found that the EORTC score overestimates the risk of recurrence and progression in patients treated with BCG. Moreover, when the EORTC data have been applied to the CUETO model, an underestimation of the risk of recurrence in low-risk patients and an overestimation of the risk of progression in high risk patients have been found (7). A multicenter European study was performed to external validate both EORTC and CUETO scores an found a poor ability of discrimination for recurrence and moderate for progression (8). Given the results of these studies, and especially the overestimation found when external data were applied to these two scores, it has been demonstrated that the EORTC and CUETO models are valid tools, but they probably need to be updated and recalculated with other variables such as the performance of re-transurethral resection (TURBT), early instillations, chemotherapy, complete BCG schedules and 2004 WHO grading classification system.
Other risk tables
Before the development of EORTC and CUETO risk scores other models of risk-stratification were proposed. In 1989 the British Medical Research Council (9) found variables related to the risk of recurrence in patients with primary superficial BCa: patients were stratified into 3 groups based on the result on the endoscopic evaluation at 3 months follow up and the presence of multifocal/monofocal disease at the initial TURBT. Later, Millán-Rodríguez et al. (10) found several factors related to the risk of recurrence and progression in 1,529 patients with a new diagnosis of non-muscle invasive BCa and they stratified the population in 3 risk-groups based in number of tumors, grade and stage.
Other clinical predictors
Several studies have shown the relationship between anemia and poor oncological outcomes in patients with BCa treated with radical cystectomy (11,12). More recently this factor was studied in patients with non-muscle invasive diseases (13) and was found as well associated with increased recurrence and progression. Similar result was reported for the neutrophil-to-lymphocyte ratio (NLR).
Nomograms
In the last 15 years several nomograms to predict the risk of recurrence and progression were proposed. Shariat et al. (14) published in 2005 a nomogram developed on 2,681 patients with previous diagnosis of Ta, T1 or CIS. Each patient was evaluated with urinary cytology, cystoscopy and urinary matrix protein 22 (NMP22). In case of suspicion of BCa, patients underwent bladder biopsies. After assessing relationship between variables and recurrence with a multivariable analysis, three nomograms were developed to predict, first of all, the risk of recurrence of transitional cell carcinoma in general, secondly the risk of recurrence of Ta-T1-Tis G3 diseases and lastly the risk of recurrence of T2 tumors. Variables included in these nomograms were age, gender, NMP22 dichotomized and the result of cytology. Later Hong et al. (15) proposed a nomogram to predict disease recurrence in patients with newly diagnosed Ta, T1 BCa. 1,587 patients were included in this multicenter study between 1998 and 2002. Variables included in the nomograms were age, tumor size, number of tumors, grade, presence of concomitant CIS and administration of intravesical therapy. Pan et al. (16) developed a nomogram to predict cancer specific-and progression free survival based on age, WHO/ISUP grade, T stage and intravesical instillations. Yamada et al. (17) found that number of tumors, shape, grade, and administration of intravesical therapy were significantly associated with recurrence-free survival whereas tumor shape and grade with progression-free survival. Six factors were included in the nomogram predicting recurrence and three for the one predicting progression.
Biomarkers
Urinary fluorescent in situ hybridization (FISH)
UroVysion (Abbott Molecular Inc., Des Plaines, IL, USA) is a cytology-based test that uses FISH to detect cells with alteration of chromosomes. It was initially developed for BCa-detection but more recently he has been applied for predicting BCG-failure in non-muscle invasive BCa. Kamat et al. (18,19) published results of a prospective randomized controlled trial including more than one hundred patients submitted to FISH before and during BCG treatment, founding that patients with positive FISH during the instillation had 3- to 5-fold increased risk of developing a recurrence compared to those with a negative FISH and 5 to 13 fold increased risk of developing progression (P<0.01). Moreover, the timing of positive FISH played an important role in prediction of failure: patients with a negative FISH result at baseline, 6 weeks, and 3 months had 8.3% recurrence rate versus 48.1% of patients with a positive test at all three analyses. The phenomenon characterized by a positive FISH test with a negative cystoscopy has been called “molecular failure”.
Other molecular biomarkers
Several biomarkers were studied as predictors of the potential response in patients with non-muscle invasive BCa treated with intravesical instillation. The most studied biomarker is p53 and several studies have analyzed its role in BCa on the basis of previous findings reporting anomalies of p53-expression in other type of neoplasms. The majority of the studies found that the overexpression of this gene was associated with progression but not with recurrence. However, as shown in a metanalysis (20), the results were conflicting, probably because of the differences in design of the study, criteria of inclusion, techniques of tissue sampling and analyses and different definition of progression, and consequently the role of p53 in BCa is still not clear. The role of retinoblastoma protein (pRb) was analyzed in patients with non-muscle invasive BCa and an altered expression of pRB was found associated with recurrence and progression after treatment with BCG alone or BCG plus interferon-alpha (IFN-alpha) (21,22). Even the level of survivin (an apoptosis inhibitor) in urine, was found associated with recurrence in patients treated with BCG or mitomycin C: the presence of urinary surviving one month after finishing intravesical instillation predicts tumor recurrence with 100% sensitivity and 78% specificity. Moreover, the specificity increases to 92% after one year from the completion of the treatment (23). Other molecules have been studied included bcl-2, E-cadherin, Ki67, CK20 and ezrin but in conclusion, none of them have been approved for the detection of recurrence or progression after intravesical instillation since the studies were very heterogenous and reported conflicting results.
Cytokines and immunological markers
Several cytokines were evaluated as predictors of response to intravesical BCG. The most studied one is interleukin (IL)-2. It has been demonstrated that reduced levels of IL-2 are related to risk of recurrence, progression, leukocyturia and adverse events in patients treated with BCG for CIS (24). Other urinary cytokines have been found as potential predictors of response to BCG treatment, such as IL-8 and 18 (25), IL-6/10 ratio (26,27), tumor necrosis factor (TNF)-alfa (28), IL-12 (29), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (30,31). However, cytokines’ urinary concentration can be altered by urinary tract infections, inflammatory processes and systemic infections therefore there are potential pitfalls by using these molecules.
Programmed death-ligand 1 (PD-L1) is a transmembrane protein ligand which can be found on tumor cells whereas its receptor, is expressed on the surface of the T cell: their bond determines the deactivation of T cells. High levels of PD-L1 were found in BCG granulomata patients failing BCG treatment (32). However, a recent study did not find a significant association between PD-L1 positivity and outcomes in T1 high grade non-muscle-invasive BCa after BCG induction therapy (33). Therefore, HLA-G is an immune checkpoint molecule that is commonly neo-expressed by solid tumors, included BCa. Its main receptor is ILT2/CD85j and it is expressed on some T lymphocytes: the bond between the ligand and its receptor suppresses the function of natural-killer cells, dendritic cells, cytotoxic T lymphocytes, CD4+ T lymphocytes and the proliferation of T cells. A recent study (34) analyzed the role of ILT2 on recurrence in non-muscle invasive BCa. This study reported a significant association between the proportion of circulating CD8+ILT2+ T cells and risk of recurrence. The role of other several immunological markers on prediction od recurrence and progressions have been tested (29,35-37). Studies evaluating the role of cytokines and immunological markers are summarized in Table 1.
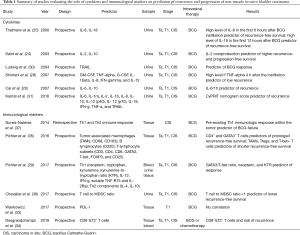
Full table
Gene based biomarkers
Several studies evaluated genetic biomarkers as predictors of recurrence and progression of non-muscle invasive BCa, and these genes were tested in combination or not with clinicopathologic characteristic to assess a risk-stratification. Pichler et al. (35) found several genetic polymorphisms involved in BCG mechanism of action analyzing data of 204 patients who underwent BCG instillations and some of them were found related to risk of progression. Moreover, a risk-score model based on the result of this study was built, and an area under the curve of 82% was found when these genes were added to clinicopathologic features (35). Dyrskjøt et al. (38) evaluated the role of 12 gene: a 12-gene progression score was built and it was found as independent prognostic factor beyond clinical and histopathological risk factors. Another study found the role of 42 genetic polymorphisms which were used to develop a risk to predict BCG response (39). Polymorphisms impairing cellular DNA damage repair (DDR) showed a significant relationship with recurrence in patients treated with BCG (40). This result was confirmed by a recent study, which showed relationship between DDR gene alterations (in particular ARID1A mutations) and the increase risk of recurrence after BCG (39). Moreover, GSTP1 and GSTO1 were found associated to recurrence in patients treated with intravesical epirubicin but not with those treated with mytomicin C (41).
CUETO and EORTC risk tables in combination with biomarkers
Several markers have been integrated to EORTC and CUETO scores. The molecular grade based on fibroblast growth factor receptor 3 (FGFR3) gene mutation status and MIB-1 expression have been tested in a multicenter study which included data of 230 patients with non-muscle invasive bladder tumors (42): the addition of the molecular grade increased the accuracy of the EORTC score from 74.9% to 81.7% (P<0.001). On the contrary, the addition of five biomarkers (p21, p27, p53, KI-67, and cyclin E1) to both CUETO and EORTC scores demonstrated only a little benefit (43) and the biomarker status was not associated to recurrence and progression.
Conclusions
EORTC and CUETO score remain the most used tools to predict recurrence, progression and treatment response, in non-muscle invasive BCa. The accuracy of both scores is currently low and an update with the newest recommendation of guidelines is needed. Several immunological, genetic and molecular biomarkers have been more recently tested and have shown to have a role as tools in prediction of non-muscle invasive BCa. Future perspectives include the combination between clinical-pathological features and molecular biomarkers to facilitated and improve patient stratification.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5; discussion 475-7.
- Mostofi FK, Sobin LH, Torloni H, et al. Histological Typing of Urinary Bladder Tumours. Geneva: World Health Organization, 1973.
- Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carci-noma of the bladder. Br J Cancer 2013;109:1460-6. [Crossref] [PubMed]
- Fernandez-Gomez J, Madero R, Solsona E, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol 2011;60:423-30. [Crossref] [PubMed]
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance bacillus Calmette-Guérin. Eur Urol 2016;69:60-9. [Crossref] [PubMed]
- Vedder MM, Márquez M, Bekker-Grob EW, de , et al. Risk prediction scores for re-currence and progression of non-muscle invasive bladder cancer: an international validation in primary tumours. PLoS One 2014;9:e96849. [Crossref] [PubMed]
- Parmar MKB, Freedman LS, Hargreave TB, et al. Prognostic Factors for Recurrence and Followup Policies in the Treatment of Superficial Bladder Cancer: Report from the British Medical Research Council Subgroup On Superficial Bladder Cancer (Urological Cancer Working Party). J Urol 1989;142:284-8. [Crossref] [PubMed]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al. Primary superfi-cial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 2000;164:680-4. [Crossref] [PubMed]
- Moschini M, Bianchi M, Gandaglia G, et al. The Impact of Perioperative Blood Transfusion on Survival of Bladder Cancer Patients Submitted to Radical Cystec-tomy: Role of Anemia Status. Eur Urol Focus 2016;2:86-91. [Crossref] [PubMed]
- Xia L, Guzzo TJ. Preoperative Anemia and Low Hemoglobin Level Are Associated With Worse Clinical Outcomes in Patients With Bladder Cancer Undergoing Radi-cal Cystectomy: A Meta-Analysis. Clin Genitourin Cancer 2017;15:263-72.e4. [Crossref] [PubMed]
- Soria F, Moschini M, Abufaraj M, et al. Preoperative anemia is associated with dis-ease recurrence and progression in patients with non-muscle-invasive bladder cancer. Urol Oncol 2017;35:113.e9-14. [Crossref]
- Shariat SF, Zippe C, Ludecke G, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or cis transitional cell carcinoma of the bladder. J Urol 2005;173:1518-25. [Crossref] [PubMed]
- Hong SJ, Cho KS, Han M, et al. Nomograms for Prediction of Disease Recurrence in Patients with Primary Ta, T1 Transitional Cell Carcinoma of the Bladder. J Korean Med Sci 2008;23:428-33. [Crossref] [PubMed]
- Pan CC, Chang YH, Chen KK, et al. Constructing prognostic model incorporating the 2004 WHO/ISUP classification for patients with non-muscle-invasive urothelial tumours of the urinary bladder. J Clin Pathol 2010;63:910-5. [Crossref] [PubMed]
- Yamada T, Tsuchiya K, Kato S, et al. A pretreatment nomogram predicting recur-rence- and progression-free survival for nonmuscle invasive bladder cancer in Japa-nese patients. Int J Clin Oncol 2010;15:271-9. [Crossref] [PubMed]
- Kamat AM, Willis DL, Dickstein RJ, et al. A novel FISH-based definition of BCG failure to enhance recruitment into clinical trials of intravesical therapies. BJU Int 2016;117:754-60. [Crossref] [PubMed]
- Kamat AM, Dickstein RJ, Messetti F, et al. Use of Fluorescence in situ Hybridiza-tion to Predict Patient Response to BCG Therapy for Bladder Cancer: Results of a Prospective Trial. J Urol 2012;187:862-7. [Crossref] [PubMed]
- Schmitz-Dräger BJ, Goebell PJ, Ebert T, et al. p53 immunohistochemistry as a prognostic marker in bladder cancer. Playground for urology scientists? Eur Urol 2000;38:691-9; discussion 700. [Crossref] [PubMed]
- Cormio L, Tolve I, Annese P, et al. Retinoblastoma protein expression predicts re-sponse to bacillus Calmette-Guérin immunotherapy in patients with T1G3 bladder cancer. Urol Oncol 2010;28:285-9. [Crossref] [PubMed]
- Esuvaranathan K, Chiong E, Thamboo TP, et al. Predictive value of p53 and pRb expression in superficial bladder cancer patients treated with BCG and interfer-on-alpha. Cancer 2007;109:1097-105. [Crossref] [PubMed]
- Hausladen DA, Wheeler MA, Altieri DC, et al. Effect of intravesical treatment of transitional cell carcinoma with bacillus Calmette-Guerin and mitomycin C on uri-nary survivin levels and outcome. J Urol 2003;170:230-4. [Crossref] [PubMed]
- Saint F, Kurth N, Maille P, et al. Urinary IL-2 assay for monitoring intravesical ba-cillus Calmette-Guérin response of superficial bladder cancer during induction course and maintenance therapy. Int J Cancer 2003;107:434-40. [Crossref] [PubMed]
- Thalmann GN, Sermier A, Rentsch C, et al. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol 2000;164:2129-33. [Crossref] [PubMed]
- Cai T, Mazzoli S, Meacci F, et al. Interleukin-6/10 ratio as a prognostic marker of recurrence in patients with intermediate risk urothelial bladder carcinoma. J Urol 2007;178:1906-11; discussion 1911-2.
- Cai T, Nesi G, Mazzoli S, et al. Prediction of response to bacillus Calmette-Guérin treatment in non-muscle invasive bladder cancer patients through interleukin-6 and interleukin-10 ratio. Exp Ther Med 2012;4:459-64. [Crossref] [PubMed]
- Shintani Y, Sawada Y, Inagaki T, et al. Intravesical instillation therapy with bacillus Calmette-Guérin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guérin immunotherapy. Int J Urol 2007;14:140-6. [Crossref] [PubMed]
- Pichler R, Gruenbacher G, Culig Z, et al. Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunol Immunother 2017;66:427-40. [Crossref] [PubMed]
- Ludwig AT, Moore JM, Luo Y, et al. Tumor necrosis factor-related apopto-sis-inducing ligand: a novel mechanism for bacillus Calmette-Guérin-induced anti-tumor activity. Cancer Res 2004;64:3386-90. [Crossref] [PubMed]
- Kamat AM, Briggman J, Urbauer DL, et al. Cytokine Panel for Response to In-travesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to bacillus Calmette-Guérin. Eur Urol 2016;69:197-200. [Crossref] [PubMed]
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carci-noma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007;109:1499-505. [Crossref] [PubMed]
- Wankowicz SAM, Werner L, Orsola A, et al. Differential Expression of PD-L1 in High Grade T1 vs Muscle Invasive Bladder Carcinoma and its Prognostic Implica-tions. J Urol 2017;198:817-23. [Crossref] [PubMed]
- Desgrandchamps F, LeMaoult J, Goujon A, et al. Prediction of non-muscle-invasive bladder cancer recurrence by measurement of checkpoint HLAG’s receptor ILT2 on peripheral CD8+ T cells. Oncotarget 2018;9:33160-9. [Crossref] [PubMed]
- Pichler R, Fritz J, Zavadil C, et al. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget 2016;7:39916-30. [Crossref] [PubMed]
- Chevalier MF, Trabanelli S, Racle J, et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest 2017;127:2916-29. [Crossref] [PubMed]
- Nunez-Nateras R, Castle EP, Protheroe CA, et al. Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol 2014;32:45.e23-30. [Crossref] [PubMed]
- Dyrskjøt L, Reinert T, Algaba F, et al. Prognostic Impact of a 12-gene Progression Score in Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Valida-tion Study. Eur Urol 2017;72:461-9. [Crossref] [PubMed]
- Pietzak EJ, Bagrodia A, Cha EK, et al. Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur Urol 2017;72:952-9. [Crossref] [PubMed]
- Gu J. Nucleotide Excision Repair Gene Polymorphisms and Recurrence after Treat-ment for Superficial Bladder Cancer. Clin Cancer Res 2005;11:1408-15. [Crossref] [PubMed]
- Deng X, Yang X, Cheng Y, et al. GSTP1 and GSTO1 single nucleotide polymor-phisms and the response of bladder cancer patients to intravesical chemotherapy. Sci Rep 2015;5:14000. [Crossref] [PubMed]
- van Rhijn BW, Zuiverloon TC, Vis AN, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol 2010;58:433-41. [Crossref] [PubMed]
- Passoni N, Gayed B, Kapur P, et al. Cell-cycle markers do not improve discrimina-tion of EORTC and CUETO risk models in predicting recurrence and progression of non-muscle-invasive high-grade bladder cancer. Urol Oncol 2016;34:485.e7-14. [Crossref] [PubMed]


