Dorsal buccal graft urethroplasty in female urethral stricture disease: a multi-center experience
Introduction
Female urethral stricture is an under-recognized condition and estimates as to its true burden vary. Older studies have put estimates of prevalence at 3–8% of all women, or 4–18% of women with bladder outlet obstruction (1-3). One more recent study analyzing a nationally-represented dataset showed the annual rate of physician office visits for female urethral stricture to be 186 per 100,000 (0.19%), with increasing prevalence among women over 65 years of age (4). The etiology is typically due to iatrogenic, traumatic, or idiopathic causes (5).
Diagnosis can be difficult due to wide and varying presenting symptoms, including dysuria, urgency, frequency, straining, incomplete emptying or urinary retention (6,7). Treatment for female urethral strictures has traditionally been with dilation and/or self-catheterization; however, there has been an increasing use of urethroplasty due to improved success rates (8-15). Urethral dilation success rates (including post-dilation self-catheterization) range from 14–49%, with a recent review reporting a weighted mean success rate of 47% with long-term follow-up (5,16,17). Comparatively, success rates for urethroplasty range from 73–100% for vaginal or labial or flap urethroplasty (mean estimates 80% and 91%, respectively) and 50–100% for oral mucosal graft urethroplasty (mean estimate 94%) (5).
Surgical techniques for female urethroplasty vary, with grafts or flaps being utilized and reconstruction performed at the ventral or dorsal urethra. As a result, female urethroplasty outcomes data is often limited by small sample sizes, single institution studies, disparate techniques of reconstruction, and varied methods to assess surgical success. Reconstruction with oral mucosal graft has shown excellent success in the repair of female urethral strictures to-date with the largest series evaluating 15 patients with 12 month follow-up data (15). Other published series of female dorsal onlay buccal mucosa graft urethroplasty have ranged from 1–8 patients precluding the ability to truly understand expected success rates among such a limited patient cohort (9,12,14,18-21). In order to augment outcome data in this area, we aimed to describe a multi-institutional experience with female dorsal-onlay buccal mucosa graft (FD-BMG) urethroplasty.
Methods
We retrospectively identified 39 consecutive dorsal BMG urethroplasties performed by 6 high-volume reconstructive surgeons at separate institutions from 12/2007 to 1/2016. Patients were included if they had at least 12 months of follow-up after their repair and a post-urethroplasty cystoscopy. Exceptions to the study inclusion requirement of 12 months follow-up were two patients who experienced surgical failure before 12 months of the urethroplasty date. Institutional Review Board approval was obtained at all sites for retrospective medical record review.
Stricture location was defined by surgeon at the proximal, mid, and/or distal urethra for each stricture. Surgical technique included dorsally-placed buccal mucosal grafts in all cases. All surgeons used a similar operative technique. The patient was placed in the dorsal lithotomy position, and the urethra was catheterized with a 10 Fr urethral catheter, if possible. If unable, a smaller size catheter or guidewire was placed across the urethra. An incision was performed above the meatus and sharp dissection was performed along the 12:00 portion of the urethra beyond the proximal extent of the urethral stricture. A dorsal urethrotomy incision was performed from the meatus beyond the proximal extent of the urethral stricture into normal urethral mucosa. Additional proximal dissection was performed until a 28–30 Fr bouge a boule sound passed without resistance. A 1.5 cm wide buccal mucosa graft was then harvested with the buccal length determined by individual stricture length. The buccal mucosa graft was then defatted, and the graft bed was periodically aerated with an 18 gauge needle to improve capillary imbibition and inosculation of the graft. Interrupted 5/0 monofilament absorbable suture was used to inlay the graft at the proximal urethrotomy opening and along the lateral edges. Periodic 5/0 monofilament absorbable sutures were placed in the graft bed to aid graft proximity to the underlying tissue. Following surgery, a urethral catheter was inserted in all patients for a median of 15 days.
Postoperative evaluation was conducted according to individual surgeons’ protocol and included postoperative cystoscopy for all. The majority of surgeons had a postoperative protocol of uroflow, PVR, and cystoscopy within the first 3–6 months after repair, followed by at least one more evaluation with uroflow, PVR and cystoscopy within 6–12 months after repair. Stricture recurrence was defined by the inability to pass a 17 Fr cystoscope.
Univariate statistical analysis was performed to compare patient, stricture, and surgery characteristics between patients with recurrence and those without recurrence. Analysis included analysis of variance for continuous predictors and chi square test with Fisher’s exact test given small sample sizes for categorical predictors, with a P value of <0.05 considered significant.
Results
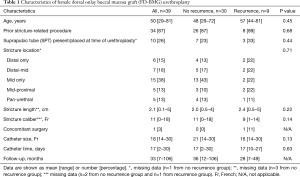
Thirty-nine women met criteria for inclusion (Table 1). The mean age at the time of surgery was 50 (range, 29–81) years. Presenting symptoms included obstructive voiding symptoms (82%), urinary frequency (67%), dysuria (62%), urgency (56%), urinary tract infection (38%), incontinence (28%), urinary retention (26%), bladder pain (8%), dyspareunia/pelvic pain (8%), and hematuria (5%). Eleven (29%) reported pre-operative incontinence (3/11 stress, 7/11 urge, 1/11 mixed).
Full table
Diagnosis was conducted by each surgeon according to individual diagnostic techniques, and diagnostic tests included cystoscopy (26 patients, 67%), post-void residual (PVR) (19 patients, 49%), uroflow (16 patients, 41%), voiding cystourethrogram (VCUG) (15 patients, 38%), bougie urethral calibration (6 patients, 15%), and urodynamics (5 patients, 13%). The mean maximum preoperative flow rate was 10.2 mL/s, and the mean preoperative PVR was 157.6 mL. Stricture etiology was unknown (49%), iatrogenic (36%), or trauma/straddle injury (15%). A majority of women (87%) women had undergone a prior stricture-related urethral procedure(s) before the surgeons’ index urethroplasty: dilation (87%), urethrotomy/bladder neck incision (21%), intermittent catheterization (18%), and urethroplasty (5%).
Intraoperatively, the stricture location was confined to the distal urethra in 6 women (15%), the mid urethra in 15 women (38%), spanned the distal-mid urethra in 7 women (18%), spanned the mid-proximal urethra in 5 women (13%), was pan-urethral in 5 women (13%), and unspecified in one woman. Mean stricture length was 2.1 (range, 0.1–5) cm and mean stricture caliber was 11 (range, 0–18) Fr. One woman underwent concomitant surgery, which was an anti-incontinence procedure for preoperative stress incontinence. The mean size of catheter that was left in place after surgery was 18 (range, 14–30) Fr, with mean catheter duration of 17 (range, 2–30) days.
Mean postoperative follow-up was 33 (range, 7–106) months. There were two patients who experienced failure at 3 months and did not follow-up beyond 7 and 8 months after FD-BMG urethroplasty. Postoperative evaluation included flow rate in 23 women (59%), VCUG in 21 women (54%), and cystoscopy in all. The mean post-operative flow rate among those studied was 22.0 mL, and this was obtained at a mean follow-up of 15.1 months. The mean post-void residual among those studied was 74.0 at a mean follow-up of 13.2 months. The 30-day postoperative complications were all urinary tract infections (7 patients, 18%). No patients developed de novo incontinence during follow-up.
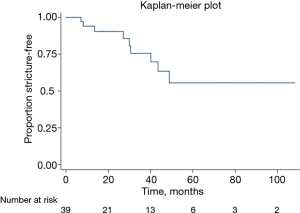
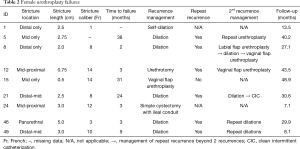
Twenty-three of women had stricture recurrence identified on follow-up, with mean time to recurrence 14.1 (range, 2–38) months (Figure 1, Table 2). Six women underwent dilation or urethrotomy, all of whom ultimately had repeat recurrences. One woman underwent vaginal flap urethroplasty with a successful outcome at 14 months of follow-up after her second surgery. One woman had stricture recurrence associated with urinary retention. Urodynamics confirmed detrusor hypocontractility, and she was subsequently managed with self-dilation. The last patient had recurrence associated with vesicovaginal fistula and persistent stress incontinence and ultimately underwent a simple cystectomy with ileal conduit. In evaluating pre- and intraoperative characteristics as risk factors for stricture recurrence, there were no characteristics identified as significant predictors for recurrence (Table 1).
Full table
Discussion
Female urethral stricture disease is relatively rare, and thus outcomes data for female urethral reconstruction are limited. Furthermore, techniques for female urethroplasty are diverse and include vaginal and labial flaps or oral grafts which can be placed via a ventral, dorsal, or circumferential approach. Unfortunately most of the published data are from small series and often includes various techniques for reconstruction (22). This leads to heterogeneity of outcomes data, with difficulty assessing outcomes of any single approach.
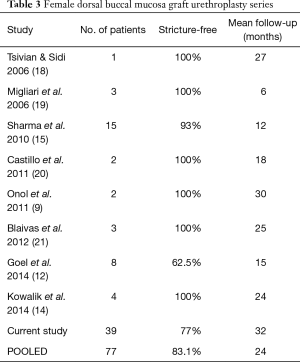
We present the largest series to-date focused solely on FD-BMG urethroplasty with a 77% success rate at a mean follow-up of 32 months. In those without recurrence, mean follow-up was 33 months. Other series have reported outcomes of FD-BMG urethroplasty with 62.5–100% success rates and mean follow-up ranging from 6–30 months (9,12,14,15,18-21). Our success rate may be somewhat lower than other studies given our longer-term follow-up and strict definition of success, as well as the high percentage of recurrent strictures being intervened upon (87% of women had previously undergone a stricture-related procedure). Further, mean time to recurrence in our series was 14 months, and most published studies never reach a mean of 14 months of follow-up. Adding our own data to previous series, the pooled success rate of FD-BMG urethroplasty is 83% among 77 individuals treated, with a mean follow-up of 24 months (Table 3).
Full table
In our series, there were no preoperative factors that significantly predicted recurrence, although those with recurrence had a slightly longer mean stricture length and smaller mean stricture caliber. We postulate that recurrent endoscopic procedures would impact urethral reconstruction; however, the majority of women had repeated endoscopic procedures and prevented assessment of this risk factor. Those without recurrence were left with a slightly larger mean catheter size, although this was not a significant finding.
Upon evaluation of other series that report failures, Sharma et al. report that in the one failure in their series, the time to failure was 3 months and this patient required repeated dilations for several months but was ultimately stricture-free at 12 months (15). In the Goel et al. series, 3 of 8 patients had recurrence, and all were treated with dilation and subsequently required self-catheterization to maintain patency (12). These results suggest that endoscopic management of recurrent female urethral strictures after urethroplasty is not likely to be successful. In our series, we were able to salvage initial failures with repeat urethroplasty in select patients, thus avoiding the need for chronic intermittent self-catheterization.
Importantly, there were no episodes of de novo incontinence, even among proximally-located strictures. This echo other data showing that de novo incontinence does not typically occur with female urethroplasty (5,22). The female urethra consists of an inner circular and outer longitudinal layer composed of muscular layers, and the external striated muscle sphincter is shaped like a horseshoe that is thickest on the ventral aspect of the urethra and thin or absent on the dorsal aspect of the urethra (8,23,24). In addition, in the female there are two arches of striated muscle located in the distal urethra at the urogenital membrane called the compressor urethrae and urethrovaginal sphincter, which help in maintaining continence (25). This anatomy allows dorsal dissection along the clitoral body in a plane where there are few fibers of the striated muscle sphincter. This, in combination with another continence mechanism distally, may help to preserve continence for these women despite reconstruction extending all the way to the proximal urethra and/or bladder neck.
Nearly all women in our series had had previous treatment before presenting to their reconstructive surgeon, and this may be related to difficulty in the diagnosis of female stricture disease, and/or providers relying on treatment modalities with poor success. A very high percentage of women had undergone previous dilation, which is consistent with the literature showing a high prevalence of dilation(s) for women with urethral stricture despite its poor success rates (4,5). The differential in success between dilation and urethroplasty intimates a gap in care, where females with urethral stricture are either not referred, or do not have access to reconstructive surgeons who are able to perform urethral reconstruction.
Another aspect of female stricture treatment that introduces inconsistency into interpreting outcomes data is the variability by which patients are both assessed before and after urethral reconstruction. In our own series, the majority of surgeons assessed pre-operative uroflow and post-void residual and performed cystoscopy and/or bougie calibration before or at the time of surgery. Some surgeons perform a voiding cystourethrogram at the time of catheter removal, whereas others perform a voiding trial alone. All of the surgeons in our series standardly perform cystoscopy within the first 3 months after repair and then again between 6 and 12 months after repair. Standardizing the approach to evaluation and follow-up after female urethroplasty will help to be able to compare outcomes data in the future in order to pool data from small series.
Finally, it is important to note that there is a lack of quality of life data surrounding female urethral strictures and their reconstruction. Data on sexual function, urinary symptoms and quality of life, and overall quality of life before and after reconstruction is necessary to understand the burden of stricture disease and the effect of urethroplasty on quality of life in these women. PROMIS® represents a set of freely available, validated patient-reported outcome measures directed towards physical, mental, and social health that was developed and evaluated with National Institutes of Health funding. Measures such as the global health, psychosocial illness impact, female sexual function and satisfaction PROMIS questionnaires are worthwhile questionnaires that are already existing, freely available, and available in both English and Spanish. Questionnaires such as these could be given to patients both before and after reconstruction to understand the impact of surgery, as well as to patients who undergo other interventions (such as dilations) to compare the change in quality of life between treatment modalities.
As this is a retrospective review with a relatively small sample size, our data is limited in its interpretation. In response to possible criticism, we hypothesize that the lower success of dorsal onlay urethroplasty in women is limited by smaller urethral length than in men. This impacts the ability to insert the buccal graft beyond the strictured urethra and into healthier urethral mucosa. Perhaps, our success rate would be higher in the setting of focal strictures that have not been impacted by repeated dilations or a long history of self-catheterization that can result in a longer urethral stricture. As such, we caution readers against comparing urethral graft success rates in men and women. We view this series as further exploration into how to better successfully manage women with urethral strictures. By restricting our population to a single repair type and approach, and given the length of follow-up in our series, we believe that our data can add to the existing literature. Because our data represent the outcomes of multiple surgeons, this does introduce variability in terms of some aspects of surgical technique, assessment and follow-up of patients that must also be considered. We view this as a strength in combining smaller single-surgeon series into a larger data pool to assess outcomes. We look forward to future publications using larger patient cohorts to augment the existing literature and hope that this publication will encourage consideration of urethroplasty in appropriate patients.
Conclusions
Female dorsal BMG urethroplasty is a safe and effective management option for female urethral stricture. Women should be referred to centers where female urethroplasty is performed for surgical consideration, rather than undergo repeated urethral dilations that have poor long-term success.
Acknowledgements
Funding: Funding for Dr. Hampson’s effort supported by NIH/NIDDK Award K12K083021.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was obtained at all sites for retrospective medical record review (IRB number: HSD 43223).
References
- Carr LK, Webster GD. Bladder outlet obstruction in women. Urol Clin North Am 1996;23:385-91. [Crossref] [PubMed]
- Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol Urodyn 2000;19:553-64. [Crossref] [PubMed]
- Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol 1999;161:1535-40. [Crossref] [PubMed]
- Santucci RA, Payne CK, Anger JT, et al. Office dilation of the female urethra: a quality of care problem in the field of urology. J Urol 2008;180:2068-75. [Crossref] [PubMed]
- Osman NI, Mangera A, Chapple CR. A systematic review of surgical techniques used in the treatment of female urethral stricture. Eur Urol 2013;64:965-73. [Crossref] [PubMed]
- Keegan KA, Nanigian DK, Stone AR. Female urethral stricture disease. Curr Urol Rep 2008;9:419-23. [Crossref] [PubMed]
- Hoag N, Chee J. Surgical management of female urethral strictures. Transl Androl Urol 2017;6:S76-80. [Crossref] [PubMed]
- Faiena I, Koprowski C, Tunuguntla H. Female Urethral Reconstruction. J Urol 2016;195:557-67. [Crossref] [PubMed]
- Onol FF, Antar B, Kose O, et al. Techniques and results of urethroplasty for female urethral strictures: our experience with 17 patients. Urology 2011;77:1318-24. [Crossref] [PubMed]
- Petrou SP, Rogers AE, Parker AS, et al. Dorsal vaginal graft urethroplasty for female urethral stricture disease. BJU Int 2012;110:E1090-5. [Crossref] [PubMed]
- Onol FF, Onol SY, Tahra A, et al. Ventral inlay labia minora graft urethroplasty for the management of female urethral strictures. Urology 2014;83:460-4. [Crossref] [PubMed]
- Goel A, Paul S, Dalela D, et al. Dorsal onlay buccal mucosal graft urethroplasty in female urethral stricture disease: a single-center experience. Int Urogynecol J 2014;25:525-30. [Crossref] [PubMed]
- Rehder P, Glodny B, Pichler R, et al. Dorsal urethroplasty with labia minora skin graft for female urethral strictures. BJU Int 2010;106:1211-4. [Crossref] [PubMed]
- Kowalik C, Stoffel JT, Zinman L, et al. Intermediate outcomes after female urethral reconstruction: graft vs flap. Urology 2014;83:1181-5. [Crossref] [PubMed]
- Sharma GK, Pandey A, Bansal H, et al. Dorsal onlay lingual mucosal graft urethroplasty for urethral strictures in women. BJU Int 2010;105:1309-12. [Crossref] [PubMed]
- Smith AL, Ferlise VJ, Rovner ES. Female urethral strictures: successful management with long-term clean intermittent catheterization after urethral dilatation. BJU Int 2006;98:96-9. [Crossref] [PubMed]
- Romman AN, Alhalabi F, Zimmern PE. Distal intramural urethral pathology in women. J Urol 2012;188:1218-23. [Crossref] [PubMed]
- Tsivian A, Sidi AA. Dorsal graft urethroplasty for female urethral stricture. J Urol 2006;176:611-3; discussion 3. [Crossref] [PubMed]
- Migliari R, Leone P, Berdondini E, et al. Dorsal buccal mucosa graft urethroplasty for female urethral strictures. J Urol 2006;176:1473-6. [Crossref] [PubMed]
- Castillo OA, Sepulveda F, Feria-Flores MA. Urethroplasty with dorsal oral mucosa graft in female urethral stenosis. Actas Urol Esp 2011;35:246-9. [Crossref] [PubMed]
- Blaivas JG, Santos JA, Tsui JF, et al. Management of urethral stricture in women. J Urol 2012;188:1778-82. [Crossref] [PubMed]
- Osman NI, Chapple CR. Contemporary surgical management of female urethral stricture disease. Curr Opin Urol 2015;25:341-5. [PubMed]
- Kim RJ, Kerns JM, Liu S, et al. Striated muscle and nerve fascicle distribution in the female rat urethral sphincter. Anat Rec (Hoboken) 2007;290:145-54. [Crossref] [PubMed]
- Elbadawi A. Functional anatomy of the organs of micturition. Urol Clin North Am 1996;23:177-210. [Crossref] [PubMed]
- DeLancey JO. Structural aspects of the extrinsic continence mechanism. Obstet Gynecol 1988;72:296-301. [PubMed]