
Fournier’s gangrene: a modern analysis of predictors of outcomes
Introduction
Fournier’s gangrene (FG) is an aggressive, rapidly progressing, necrotizing fasciitis of the perineal and genital region. The disease initially described by Alfred Fournier in 1883 was a polymicrobial life threatening infection of unknown origin occurring in otherwise healthy young men (1). Mortality rates were initially reported in the range of 20–30%, which remains the accepted textbook mortality (2-6). However, this disease is now known to occur in a wide age range, frequently in older patients, and usually with an identifiable infectious source. Urinary extravasation, perirectal and periurethral skin infections serve as the common nidus of infection. Diabetes, immunosuppressed states and obesity often contribute to its rapid progression (7,8).
Despite the advantage of a known etiology, evidence-based management is still challenging. Because of wide variability in presentation, clinical course, and mortality rates, it can be difficult to predict which patients warrant the most aggressive approach.
In an effort to risk stratify these patients, Laor et al. devised the FG Severity Index Score (FGSI) to predict mortality in patients with FG. The FGSI is a scoring system that consists of 9 lab parameters and vital signs, measured at presentation, each assigned a score from 0 to 4 based on deviation from the normal range. Specifically, Laor et al. found a dramatic increase in mortality (from 22% to 75%) once the FGSI score rose above 9 (9). In the intervening 20 years, several case series have attempted to validate the predictive utility of this score with mixed results.
Based on an extensive review of the literature, there has not been a recent case series at a level 1 trauma center in the United States that has evaluated the validity of the FGSI. Our study aims to validate the FGSI as a prognostic tool for predicting patient morbidity and mortality in this environment.
Methods
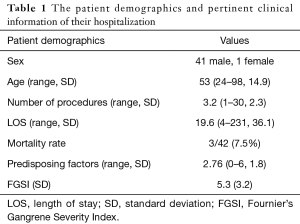
Hospital medical records were queried for diagnosis codes corresponding to FG and scrotal cellulitis. The medical records of 42 patients treated for FG at Washington Hospital Center in Washington, DC between 2001 and 2015 were identified and reviewed. Operative notes were reviewed for deliberate mention of necrotizing fasciitis or Fournier’s to confirm FG. All patients received perioperative fluid resuscitation, as well as broad spectrum antibiotic coverage pending culture results. Standard initial antibiotics within our institution include vancomycin and piperacillin-tazobactam. Use of vasopressor support was based on shared decision of the critical care, urology, and anesthesia teams. Data tabulated from the medical records included vital signs at admission, serum sodium, potassium, creatinine, hematocrit, WBC, bicarbonate. An FGSI score as described by Laor et al. was calculated for 38 of the 42 patients. Four patients were excluded due to incomplete lab results (9). Additional data seen in Table 1 including length of stay (LOS), number of operations performed during the single admission, mortality, and number of comorbidities were collected as additional prognostic factors to test.
Full table
Logistic regression analysis was performed using SAS statistical software (SAS, NC, USA) to investigate whether FGSI predicts mortality. Pearson correlation coefficients were calculated to analyze the relationship between individual variables (e.g., serum Cr, hematocrit, LOS, number of operations, comorbidities) and FGSI score. Receiver operating characteristic (ROC) curve was calculated based on Mann-Whitney testing of the relationship between mortality and FGSI. Odds ratio estimates were calculated to assess risk of mortality with each FGSI score.
Results
Of the 42 patients with confirmed FG, 3 patients died during the original admission (7.5%), and 39 survived until discharge (92.5%). Mean patient age was 53 years old, and mean LOS was 19.6 days. FGSI scores ranged from 1 to 13. All patients were treated at MedStar Washington Hospital Center, and underwent an average of 3.2 surgical procedures prior to discharge. Etiologies of FG were varied and included scrotal cellulitis, scrotal abscess, perirectal/perianal abscess, persistent urethral catheterization, hidradenitis, infected Bartholin cyst, and decubitus ulceration.
Documented comorbidities included mellitus (DM), HIV, alcohol abuse, iv drug abuse, coronary artery disease, peripheral vascular disease, chronic kidney disease, obesity, neurologic disease, hypertension, chronic obstructive pulmonary disease (COPD), congestive heart failure, malignancy, and immunosuppression.
The average number of patient comorbidities at presentation was 2.76, with the most common condition being Diabetes, which occurred in 24 of 42 (57.1%) of patients. The number of comorbidities was not associated with patient mortality.
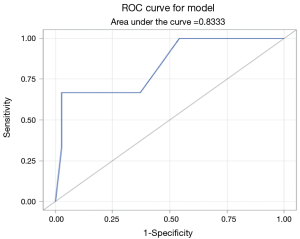
FGSI was calculated for 38 of the 42 patients. The average FGSI score was 5.2. Patients that died had an average FGSI of 10.0, where patients who survived had a mean FGSI score of 5.0. Logarithmic regression analysis showed the relationship between FGSI and mortality was statistically significant, with a Pearson correlation coefficient of 1.63 (CI: 1.04–2.49, P=0.0313) for each interval increase in FGSI score. ROC analysis showed a strong association between FGSI score and patient mortality, with area under the ROC curve equal to 0.8333 (Figure 1). There was a statistically significant relationship between FGSI and length of hospital stay (R=0.40, P=0.0121). FGSI score showed no significant relationship to number of comorbidities or to number of surgical procedures.
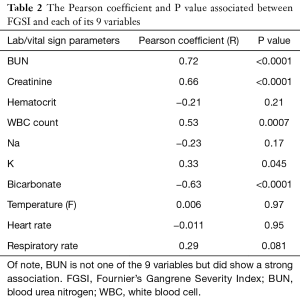
In addition to the association of comorbidities and FGSI to mortality, we measured the association between FGSI and each of its nine individual components (Table 2). There was a statistically significant association between FGSI and 4 of its 9 variables: Creatinine (R=0.66), Bicarbonate (R=−0.63), white blood cell (WBC) count (R=0.53), and Potassium (R=0.33). There was also a strong relationship between blood urea nitrogen (BUN) (not part of the FGSI score) and FGSI, with R=0.72. Increases in 5 of the 9 constituent variables were not associated with increases in FGSI.
Full table
Discussion
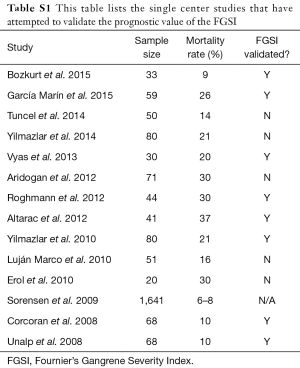
FG is a rare, life threatening disease with a clinical course that remains challenging to predict. Early identification of patients at high risk for mortality allows rapid advancement of care and may provide survival benefit. While many institutions including our own use computed tomography (CT) scans to detect free air within the soft tissues, ultrasound has been reported to be of benefit for bedside detection of free air when CT is not readily available (10). The FGSI score is the most widely used prognostic tool in the management of FG. Table S1 lists the single center studies that have attempted to validate the prognostic value of the FGSI (2-5,7,11-19).
Full table
In addition, more recently, two larges database-based studies were published in the United States showing substantially lower mortality rates of 7.5% and 10% (20,21). The mortality rate reported in this study is in line with these recent publications suggesting improved outcomes for FG patients compared to the higher mortality rates often cited in textbooks.
Based on the previous decade of experience presented here, the mortality rates of FG have improved over the past twenty years and more closely resemble the lower rates sited above of 7–10%. It is also worth noting that the FGSI is predictive of mortality and LOS in the setting of a low mortality rate. Possible reasons for a lower mortality rate include the early use of broad-spectrum antibiotics, ICU care involvement, better recognition of the disease on the primary care provider level and shorter time to debridement. A recent study compared early debridement versus conservative management of early FG (equivocal cases) and confirmed early debridement led to shorter hospital stays and better clinical outcomes (22). No studies directly attempt to identify causes of a lower mortality rate but the above study shows that clinical care is likely responsible and not that the pathophysiology of the disease has changed.
In addition to validation of the FGSI as a predictor of morbidity and mortality, our results also showed a surprising lack of association between FGSI and 5 of its 9 constituent variables. This implies that changes in these 5 variables do not correspond to an overall increase in mortality, and therefore may not add value to the scoring system. This allows for the possibility that a modified FGSI with fewer variables may yield similar, or even superior, prognostic results. A simpler evaluation system could presumably improve utilization or implementation in the clinical setting.
A recent 2014 study by Lin et al. found that a simplified scoring system using only 3 clinical variables was non-inferior to FGSI in predicting patient mortality in an 85-patient series (21). If a reliable, simplified scoring system can be developed that is easier for clinicians to calculate, the likelihood of clinical use increases. The findings of this paper further support that such a system may offer improved predictions of clinical outcomes.
Our study is not without its limitations. The small number of mortalities (3), represent a statistical limitation to the study. In addition, the retrospective nature of the study limits the possible prognostic variables to those that were recorded accurately at the time of hospitalization. Other studies have shown several other clinical factors to be associated with increased mortality. Variables such as increased surface area, delayed treatment onset, advanced age, cirrhosis, anorectal vs. penoscrotal source, and immunocompromised status have all been shown to result in increased incidence of death (11,17,23,24). Incorporating these factors into the FGSI or a newly designed scoring system may improve prognostic accuracy.
Conclusions
The overall mortality rate of 7.5% in this series is similar to more recent studies of FG and substantially lower than historical case series. FGSI score was significantly associated with patient mortality, and length of hospital stay. This scoring tool holds utility in stratifying risks and outcomes. Only 4 of 9 constituent variables of the FGSI were associated with the overall FGSI score, indicating that a simpler FGSI score may prove equally valid and easier to use.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fournier JA. Jean-Alfred Fournier 1832-1914. Gangrène foudroyante de la verge (overwhelming gangrene). Sem Med 1883. Dis Colon Rectum 1988;31:984-8. [PubMed]
- Erol B, Tuncel A, Hanci V, et al. Fournier’s gangrene: overview of prognostic factors and definition of new prognostic parameter. Urology 2010;75:1193-8. [Crossref] [PubMed]
- Aridogan IA, Izol V, Abat D, et al. Epidemiological characteristics of Fournier's gangrene: a report of 71 patients. Urol Int 2012;89:457-61. [Crossref] [PubMed]
- García Marín A, Turégano Fuentes F, Cuadrado Ayuso M, et al. Predictive factors for mortality in Fournier' gangrene: a series of 59 cases. Cir Esp 2015;93:12-7. [Crossref] [PubMed]
- Vyas HG, Kumar A, Bhandari V, et al. Prospective evaluation of risk factors for mortality in patients of Fournier's gangrene: A single center experience. Indian J Urol 2013;29:161-5. [Crossref] [PubMed]
- Cohen M. Fournier’s gangrene. AUA Update 1986;5:6.
- Wolach MD, MacDermott JP, Stone AR, et al. Treatment and complications of Fournier’s gangrene. Br J Urol 1989;64:310-4. [Crossref] [PubMed]
- Clayton MD, Fowler JE Jr, Sharifi R, et al. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet 1990;170:49-55. [PubMed]
- Laor E, Palmer LS, Tolia BM, et al. Outcome prediction in patients with Fournier’s gangrene. J Urol 1995;154:89-92. [Crossref] [PubMed]
- Dell’Atti L, Cantoro D, Maselli G, et al. Distant subcutaneous spreading of Fournier’s gangrene: An unusual clinical identification by preoperative ultrasound study. Arch Ital Urol Androl 2017;89:238-9. [Crossref] [PubMed]
- Bozkurt O, Sen V, Demir O, et al. Evaluation of the utility of different scoring systems (FGSI, LRINEC and NLR) in the management of Fournier's gangrene. Int Urol Nephrol 2015;47:243-8. [Crossref] [PubMed]
- Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier's gangrene: experience with 50 patients. Scand J Urol 2014;48:393-9. [Crossref] [PubMed]
- Yilmazlar T, Ozturk E, Ozguc H, et al. Fournier's gangrene: an analysis of 80 patients and a novel scoring system. Tech Coloproctol 2010;14:217-23. [Crossref] [PubMed]
- Yılmazlar T, Işık Ö, Öztürk E, et al. Fournier's gangrene: review of 120 patients and predictors of mortality. Ulus Travma Acil Cerrahi Derg 2014;20:333-7. [Crossref] [PubMed]
- Unalp HR, Kamer E, Derici H, et al. Fournier's gangrene: evaluation of 68 patients and analysis of prognostic variables. J Postgrad Med 2008;54:102-5. [Crossref] [PubMed]
- Altarac S, Katušin D, Crnica S, et al. Fournier's gangrene: etiology and outcome analysis of 41 patients. Urol Int 2012;88:289-93. [Crossref] [PubMed]
- Roghmann F, von Bodman C, Löppenberg B, et al. Is there a need for the Fournier's gangrene severity index? Comparison of scoring systems for outcome prediction in patients with Fournier's gangrene. BJU Int 2012;110:1359-65. [Crossref] [PubMed]
- Luján Marco S, Budía A, Di Capua C, et al. Evaluation of a severity score to predict the prognosis of Fournier's gangrene. BJU Int 2010;106:373-6. [Crossref] [PubMed]
- Corcoran AT, Smaldone MC, Gibbons EP, et al. Validation of the Fournier's gangrene severity index in a large contemporary series. J Urol 2008;180:944-8. [Crossref] [PubMed]
- Sorensen MD, Krieger JN, Rivara FP, et al. Fournier's gangrene: management and mortality predictors in a population based study. J Urol 2009;182:2742-7. [Crossref] [PubMed]
- Lin TY, Ou CH, Tzai TS, et al. Validation and simplification of Fournier's gangrene severity index. Int J Urol 2014;21:696-701. [Crossref] [PubMed]
- El-Shazly M, Aziz M, Aboutaleb H, et al. Management of equivocal (early) Fournier's gangrene. Ther Adv Urol 2016;8:297-301. [Crossref] [PubMed]
- Martinelli G, Alessandrino EP, Bernasconi P, et al. Fournier's gangrene: a clinical presentation of necrotizing fasciitis after bone marrow transplantation. Bone Marrow Transplant 1998;22:1023-6. [Crossref] [PubMed]
- Kuo CF, Wang WS, Lee CM, et al. Fournier's gangrene: ten-year experience in a medical center in northern Taiwan. J Microbiol Immunol Infect 2007;40:500-6. [PubMed]


