
The diagnosis of bladder cancer: are we missing a teachable moment for smoking cessation?
Cigarette smoking is a well-established risk factor for developing bladder cancer (BC), with as many as half of the cases being attributable to tobacco smoke exposure (1). The diagnosis of a smoking-related morbidity has been traditionally considered a special opportunity for introducing smoking cessation intervention, or a so-called “teachable moment” (2). For this reason, a new diagnosis of BC in a smoking patient could serve as this teachable moment and, if not missed, lead to clinical benefits of smoking cessation.
A recent study in The Journal of Urology by Winters et al. (3) investigated the relationship between a new diagnosis of BC and self-reported smoking cessation rates in the population of patients participating in Medicare Advantage Organizations. The authors analyzed data derived from the Medicare Health Outcomes Survey (MHOS)-Surveillance, Epidemiology and End Results (SEER) data resource, which links the results of the MHOS, which is a questionnaire administered to a sample of patients and then repeated ~2 years later to provide information on the health-related quality of life, as well as smoking status, with data collected from the SEER program, which is a source of epidemiologic information on the incidence and survival rates of cancer in the United States. A cohort of patients with new BC diagnosis and both pre- and post-diagnosis MHOS data available (n=394) were compared to non-cancer controls (n=1,970) in terms of smoking prevalence and cessation rates. The data analysis revealed that the difference in smoking cessation rates between the newly diagnosed BC patients and the non-cancer controls was low and not statistically significant (OR 1.3, 95% CI: 0.7–2.5). The results were then similar for the diagnosis of renal cell carcinoma (RCC) (n=169, OR 1.2, 95% CI: 0.5–3.6). It is worth mentioning that only 63 BC patients were smokers, and 27% quit smoking, producing a study cohort of only 17.
It is indicated in the article that the findings presented are consistent with other current literature reports, namely the study by van Osch et al. (4) and the study by Westmaas et al. (5). Nevertheless, the authors are aware of the limitations of their study, including its retrospective design and the nature of the MHOS-SEER data set. It is also suggested, that the small sample size may have contributed to small statistical power of the study and, consequently, to statistical insignificance of the results. Furthermore, limiting the study population to Medicare Advantage Organization participants might have attributed to a possible selection bias.
The authors of this commentary believe that the major limitation of the study by Winters et al., recognized in the paper, was the scarcity of information regarding the overall smoking history of involved patients, as the only smoking-related item of the MHOS questionnaire was: “Do you now smoke every day, some days, or not at all?”, and assessing this current smoking status was conducted only twice, in two distinct time points. The data on the study population did not include predictors of smoking cessation, such as duration and intensity of smoking, the strength of tobacco dependence and quit motivation, the number of past quit attempts, etc. Moreover, the lack of information regarding any unsuccessful quit attempts that might have had occurred during the two-year period before the MHOS-questionnaire was conducted for the second time, as well as not including data whether patients had been aware of the role of smoking in their disease or whether any cessation-counseling had been provided, further limits the insight into the true influence of BC diagnosis on smoking cessation rates and the motivation to quit.
It is also worth mentioning, that using self-reporting for the purpose of smoking-status assessment may have also posed some risk of bias for the outcomes of the study. While some studies show that self-reporting of cigarette smoking may underestimate the true prevalence by as little as 0.3–0.6% (6,7), in some populations the rate of inaccurate reporting of having quit smoking may be as high as 25% (8). Using the comprehensive Lifetime Interview on Smoking Trajectories may provide a reliable instrument for assessing detailed retrospective smoking history data (9). The definition of smoking/smoker varies throughout the literature. For example, some studies define current smoker as an adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes. The American College of Surgeon’s National Surgical Quality Improvement Program defines smoking as if the patient has smoked cigarettes in the year prior to admission for surgery. This has a tremendous potential to impact smoker prevalence.
Regardless of the mentioned limitations, the pessimistic results published by Winters et al. imply a need for focusing the clinical discussion predominantly on how not to miss this teachable moment of BC diagnosis for cessation of smoking. As mentioned in the discussed article, the low prevalence of smoking cessation in BC patients may be at least partially explained by inadequate awareness of links between tobacco smoke exposure and bladder carcinogenesis in the general population, with only 25–36% of patients identifying smoking as a risk factor of BC in cross-sectional studies, compared to 94–98% of responders recognizing the risk with lung cancer (10,11). Unfortunately, the limitations of MHOS-SEER data resource made Winters et al. incapable of stratifying the population by risk factor-awareness, as well as by smoking cessation intervention-status, which could have definitely provided much more insight into the study results.
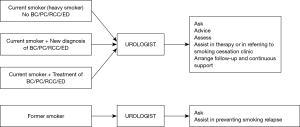
Given the established role of tobacco smoke exposure in development and natural history of many urologic diseases, especially bladder carcinoma and other urogenital cancers, as well as considering the unique intimate character of the relationship between a patient and his/her urologist, it is the urologist who should play an essential role in the process of smoking cessation (12). Every active or former smoker encountered in daily clinical practice, regardless of their level of dependence, should receive a personalized, yet explicit message from their urologist regarding the toxicity of smoking and its influence on the urologic disease presented by the patient (Figure 1).
In an earlier study, Bassett et al. (13), whose results may appear to be in contrast to the discussed study by Winters et al., after comparing survey data collected from non-muscle invasive BC patients selected from the California Cancer Registry to general population controls obtained from the California Tobacco Survey, reported that smokers with a new diagnosis of BC were almost five times as likely to quit smoking as smokers in the general population (48% vs. 10%, respectively, P<0.001). Importantly, the study population was limited to residents of California, a state known for implementing an effective tobacco control program (14). An interesting result published in the study by Bassett et al., which may be considered substantial in the context of this discussion, was that 55% of recent ex-smokers cited the advice of urologist as a reason for cessation, compared to 28% of those, who cited the advice of primary care physician.
In light of the above, the role of the urologists appears to be vital. Bjurlin et al. (15), revealed that a single 5-minute brief smoking cessation intervention performed by a urologist (regardless of the patient cancer status) was associated with an increased rate of cessation attempt (OR 2.31, P=0.038). A so-called five A’s intervention has been developed and endorsed by several public health institutions (16). The first A is for ask: every patient encountered by a urologist should be asked about their smoking status. The second A is for advice: both by authoritatively encouraging the patient to cease smoking (e.g., As your urologist I must advise you that smoking is harmful to your health and it would be important for you to stop) and by short description of the benefits of smoking cessation, both in terms of urologic and general health. The third A is for assess whether the patient is currently willing to make a quit attempt. The fourth A is for assist, which involves some minimal initial medical or behavioral intervention, or at least referring to a smoking cessation clinic. Finally, the fifth A is for arrange, which involves the urologist active help in arranging follow-up or referral. This fifth A emphasizes the role of the urologist in monitoring of the smoking cessation process. The urologist should be aware that smoking cessation is a long-lasting and multistage process, consisting of multiple failures, mainly due to stressful events and unfavorable social triggers. The optimal smoking cessation counseling should be individualized and, if necessary, rely on strict collaboration between the urologist and a specialized smoking cessation clinic (17).
Unfortunately, the expectations are not even close to the reality of daily clinical practice. A survey conducted in 2008 among the members of the American Urological Association revealed, that more than half (55.6%) of American urologists never discussed smoking cessation while only 19.8% always discussed smoking cessation with BC patients (18). Interestingly, contrary to available evidence (19), 40.7% of urologists who never discussed smoking cessation believed that smoking cessation may not alter the course or outcome of the disease. Most urologists (93.7%) had never had formal smoking cessation training and 37.7% of those who never discussed cessation did not feel qualified giving such counseling.
All the above considerations lead to a conclusion that the diagnosis of BC on many occasions still remains a missed opportunity to exploit the teachable moment for smoking cessation, and hence to improve not only urologic, but also general health outcomes. The authors of this commentary strongly believe that much more emphasis should be put on encouraging urologists to introduce smoking cessation intervention in their patients, as well as on training practitioners in effective smoking cessation-counseling.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737-45. [Crossref] [PubMed]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003;18:156-70. [Crossref] [PubMed]
- Winters BR, Wen L, Holt SK, et al. Does the Diagnosis of Bladder Cancer Lead to Higher Rates of Smoking Cessation? Findings from the Medicare Health Outcomes Survey. J Urol 2019;202:241-6. [Crossref] [PubMed]
- van Osch FHM, Jochems SHJ, Reulen RC, et al. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: a prospective cohort study. Cancer Causes Control 2018;29:675-83. [Crossref] [PubMed]
- Westmaas JL, Newton CC, Stevens VL, et al. Does a Recent Cancer Diagnosis Predict Smoking Cessation? An Analysis From a Large Prospective US Cohort. J Clin Oncol 2015;33:1647-52. [Crossref] [PubMed]
- West R, Zatonski W, Przewozniak K, et al. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev 2007;16:820-2. [Crossref] [PubMed]
- Wong SL, Shields M, Leatherdale S, et al. Assessment of validity of self-reported smoking status. Health Rep 2012;23:47-53. [PubMed]
- Gerritsen M, Berndt N, Lechner L, et al. Self-Reporting of Smoking Cessation in Cardiac Patients: How Reliable Is It and Is Reliability Associated With Patient Characteristics? J Addict Med 2015;9:308-16. [Crossref] [PubMed]
- Colby SM, Clark MA, Rogers ML, et al. Development and reliability of the lifetime interview on smoking trajectories. Nicotine Tob Res 2012;14:290-8. [Crossref] [PubMed]
- Nieder AM, John S, Messina CR, et al. Are patients aware of the association between smoking and bladder cancer? J Urol 2006;176:2405-8; discussion 2408. [Crossref] [PubMed]
- Bjurlin MA, Cohn MR, Freeman VL, et al. Ethnicity and smoking status are associated with awareness of smoking related genitourinary diseases. J Urol 2012;188:724-8. [Crossref] [PubMed]
- Sosnowski R, Bjurlin MA, Verze P, et al. Role of cigarette smoking in urological malignancies and clinical interventions for smoking cessation. Cent European J Urol 2016;69:366-9. [PubMed]
- Bassett JC, Gore JL, Chi AC, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol 2012;30:1871-8. [Crossref] [PubMed]
- Messer K, Pierce JP, Zhu SH, et al. The California Tobacco Control Program's effect on adult smokers: (1) Smoking cessation. Tob Control 2007;16:85-90. [Crossref] [PubMed]
- Bjurlin MA, Cohn MR, Kim DY, et al. Brief smoking cessation intervention: a prospective trial in the urology setting. J Urol 2013;189:1843-9. [Crossref] [PubMed]
- Quinn VP, Hollis JF, Smith KS, et al. Effectiveness of the 5-As tobacco cessation treatments in nine HMOs. J Gen Intern Med 2009;24:149-54. [Crossref] [PubMed]
- Sosnowski R, Przewoźniak K. The role of the urologist in smoking cessation: why is it important? Urol Oncol 2015;33:30-9. [Crossref] [PubMed]
- Bjurlin MA, Goble SM, Hollowell CM. Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol 2010;184:1901-6. [Crossref] [PubMed]
- Chen CH, Shun CT, Huang KH, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int 2007;100:281-6; discussion 286. [Crossref] [PubMed]


