Techniques and considerations of prosthetic surgery after phalloplasty in the transgender male
Introduction and backgroundOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
Transgender individuals have become increasingly recognized and accepted in today’s society. The worldwide prevalence is estimated to be 4.6 per 100,000 individuals, with a ratio of 2.6:1 of transgender women to transgender men (1). Recent studies estimate that 1–1.4 million Americans, about 390 per 100,000 adults, identify as transgender (2,3). Advances in governmental regulations and insurance policies have made gender-affirming surgeries more attainable.
The purpose of gender-affirming surgery is to provide transgender individuals with an outcome that aligns their physical appearance with their gender identity. Phallic reconstruction was first described by Bogoraz in 1936 (4). The techniques for phallic reconstruction have evolved and currently include free flap tissue transfers from the radial forearm, anterolateral thigh (ALT), latissimus, or fibula and local rotational flaps from the abdomen, groin, or thigh (5). While no consensus exists regarding the ideal operative technique for phalloplasty, surgeons today commonly utilize the radial artery-based forearm free flap (RFFF), with common alternatives such as ALT or latissimus available.
The optimal neophallus should be aesthetically appropriate but also functional. While standing to urinate has been recognized as a priority for most, neophallus rigidity and sexual capability has also been deemed a prime concern for 86% of patients (6). Historically, cartilage and bone transplants, splints, and malleable prostheses were used to achieve this goal; however, these techniques have largely been abandoned due to concerns surrounding persistent rigidity, high rates of failure, and pressure necrosis (7). The use of a hydraulic penile prosthesis in phalloplasty was first reported by Puckett and Montie in 1978 (8). Though this remains the most common technique of achieving rigidity in the neophallus, the ideal method of accomplishing this goal remains controversial. With reported modern complication rates ranging from 23–70%, there is clearly room for improvement in this realm (9-14). With the growing population of patients who desire these procedures, ongoing development and refinement in techniques continues by leaders in the field.
Beyond phalloplasty and penile prosthesis, scrotoplasty and testicular prostheses are an important component of creating aesthetic genitalia. Various techniques have been described to accomplish both a visually and functionally adequate scrotum in the transgender male (15-20). Unique challenges of completing a successful scrotoplasty include interference with the reconstructed neourethra, preservation of sensation, and maintenance of the scrotum in an anterior position (15). Multiple scrotoplasty techniques have been described, including pedicled skin flaps, myocutaneous flaps, free flaps, perineal advancement flaps, and labia majora skin flaps (16,17). The most commonly performed technique involves using labia majora flaps, which provide a superior cosmetic result with a more anterior position of the neo-scrotum (18-20). When utilizing the labia majora for scrotoplasty, it is important to preserve labial fat tissue in the flap to protect to future testicular implants (21).
First described in the 1940s, testicular implants have been fashioned from a wide range of materials. Today, they are mainly made of silicone rubber filled with either saline or silicone gel (22). The ideal implant is inert, creates minimal inflammatory reaction, resists mechanical strain, and feels natural for the patient.
This article provides an overview of the perioperative considerations, adaptive surgical techniques, and unique challenges faced by urologists who perform implantation of testicular and penile prostheses in individuals undergoing masculinizing genital reconstructive surgery.
Penile prosthesisOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
Differences compared to the cis-male
The key challenges of penile prosthesis placement in the neophallus are attributable to unique anatomical differences between the neophallus and native penis. First, the lack of divergent penile crura, which root the corpora cavernosa to the ischial rami, makes it difficult to anchor the proximal end of the inflatable penile prosthesis (IPP). This can often result in malposition of the prosthesis (9). Additionally, the native penis is comprised of two corpora cavernosa with enveloping tunica albuginea, which function to contain the penile implant and prevent distal protrusion. The absence of these structures as well as the muted tactile sensation in the neophallus results in significantly higher risk of distal erosion among transgender patients (9,14). Similar to patients suffering from diabetic neuropathy, the pressure of the prosthesis on the skin may not be initially noticed, which can lead to advanced skin breakdown and device erosion. The restricted vascular supply and significant scarring associated with neophallus flaps also impedes the usual healing process and increases the risk of infection and erosion (9). Finally, transgender men undergoing penile prosthesis implantation are generally younger than cis-gender men undergoing implantation for erectile dysfunction. As a result, they may be more sexually active, which is theorized to increase the rate of device failure and displacement (13). Given these anatomical variations, surgeons have developed novel strategies to minimize the resultant complications.
Timing of implantation
The implantation of a penile prosthesis is often the final step in reconstruction of the neophallus. Tactile sensation of the forearm free flap neophallus is commonly achieved by coaptation of a dorsal cutaneous branch of the pudendal nerve or other somatic nerve, such as the ilioinguinal nerve, to a recipient nerve of the flap (e.g., lateral antebrachial cutaneous, medial antebrachial cutaneous, or lateral sural cutaneous) (23,24). Some surgeons perform a second anastomosis to nerve branches of the dorsal clitoral nerve to enhance erogenous sensation. Peak tactile and erogenous feedback are often achieved 6–12 months post-operatively (13,25). Prosthesis implantation should generally be delayed until this sensation is developed to minimize the risk of implant erosion (23,26). Given the high complication rate associated with neophallus creation, this waiting period also allows the neourethra to heal and provides time for any necessary revisions to take place prior to proceeding with implantation (14). With successful nerve coaptation, return of genital sensitivity after phalloplasty is expected in most patients, with >90% of patients experiencing glans sensitivity and erogenous sensation (23). Though not described in the literature, we have also identified a small number of phalloplasty patients who complain of mild to severe graft hypersensitivity.
Operative approach
The patient should be fully healed from both phalloplasty and scrotoplasty prior to cylinder placement. If the scrotum is an inadequate size, initial placement of a tissue expander is can be performed. Falcone et al. describe a staged approach, with placement of the reservoir and a single large testicular prosthesis in the labia majora ipsilateral to the dominant hand during the glans sculpting stage. About three months later, the cylinder(s) is inserted with replacement of the testicular implant with the pump (14).
As with placement in cis-gender men, multiple insertion techniques have been described. The ultimate choice of how to approach implantation depends on surgeon preference, patient surgical history, anatomy, and prosthesis type (27). Multi-component IPPs in cis-gender men are traditionally placed through either a penoscrotal or infrapubic incision (27). Similarly, in the neophallus, both parascrotal (along the lateral aspect of the neo-scrotum) and infrapubic (along the previous phalloplasty incision) incisions have been described (9-14,25,26,28). We generally prefer an infrapubic approach. An elliptical, transverse infrapubic incision along the prior suture line is made, favoring the side contralateral to the vascular anastomosis. Dissection continues towards the midline until the pubic bone is exposed. The proximal aspect of the cylinder is then anchored to the pubic symphysis. Details of this will be discussed later. Alternatively, Zuckerman et al. describe bilateral incisions over the ischial tuberosities to reach the inferior pubic rami for anchoring (11). In all approaches, special caution should be used to avoid compromising the vascular supply of the neophallus. Continuous manual palpation of the vessels and intraoperative Doppler ultrasound can be utilized to confirm an intact vascular supply. The location of the dorsal clitoral nerve should be identified and avoided as loss of this anastomosis will lead to loss of sensation to the neophallus. Inadvertent ligation can be fixed if recognized.
Dilation and number of cylinder(s)
The surgeon and patient together must determine whether one or two cylinders will be implanted. In general, this decision is made based on the girth of the neophallus. Though placing two cylinders may increase the bulk of the neophallus, Hoebeke et al. have advised against this practice due to the resultant distortion (9,25). Additionally, the need to perform two dilations when placing dual cylinders exposes the neourethra to increased risk of damage and raises the risk of cylinder erosion. In our experience, placing a single cylinder is usually adequate and provides an acceptable aesthetic result (13).
Urethral catheterization warrants discussion. While most surgeons place a catheter during penile prosthesis surgeon in cis-males, some have abandoned this practice. In the transgender patient, prior to distal dilation, a urethral catheter may be placed allow for tactile identification of the neourethra if needed. This can be in the form of a straight catheter in the pendulous urethra only. In the setting of an ectopic reservoir placement, a completely empty bladder is not essential, thus some have abandoned catheter placement altogether. Catheterization into the bladder can be very challenging given various segments of the transgender neourethra. Efforts should be made to limit significant urethral manipulation (e.g., cystoscopic catheterization over a wire) as bacterial colonization is common, and spillage may predispose the patient to prosthetic infection. Special attention should be taken to avoid the neourethra during dilation. Distal dilation is performed with Hegar dilators to create a space with adequate width to house the cylinder. Care should also be taken to avoid distal perforation by sparing at least 1.0 cm of tissue at the distal aspect of the neophallus (9). Unlike dilation in cis-men, there is no native space homologous to the corporal bodies in the neophallus for dilation. As such, this step can be quite daunting. Because a large amount of force is sometimes required, it is critical that physicians simultaneously brace the dilator to avoid iatrogenic perforation. Alternative techniques to aid in creating this space include dilation with Brooks dilators (Coloplast, Minneapolis, MN, USA), Dilamezinsert (Cooper Surgical, Trumbull, CT, USA), or dissecting with Metzenbaum scissors. Some centers prefer to place adjuvant biologic material at the tip of the cylinder, a distal windsock graft, to induce scarring and simulate a distal corporal body. This approach is a comparable one to management of impending distal erosion in a cis-male described by some groups (29,30).
Following dilation, the appropriate cylinder length is measured. The proximal end of the cylinder is then secured using one of the various techniques discussed below.
Proximal fixation of the cylinder
Given the absence of penile crura and corpora cavernosa, there is a propensity of the cylinder to migrate and/or malrotate within the neophallus. Larger retrospective series have identified rates of cylinder malposition to be 12–20% (9,10,14). Thus, the cylinder must be anchored to either the pubic symphysis or inferior pubic rami. Various approaches to anchoring have been described.
Hoebeke et al. initially described a technique in a series of 35 patients wherein the proximal end of the prosthesis is covered with a Dacron polyester vascular graft. Subsequently, the graft-cylinder complex was secured to the pubis with polyester suture (25). Hoebeke later abandoned this approach, citing high rates of prosthesis dysfunction secondary to cylinder erosion. His contemporary technique, described in a follow up series of 129 patients, involves covering the cylinder base with a rear tip extender and securing this to the pubis with non-absorbable suture (9). Neuville et al. describe a similar approach, where the proximal cylinder is covered in a different polyester graft, Hemashield GOLD® (Maquet, Rastatt, Germany) (10).
Another method of anchoring is to fashion a full windsock or neotunica albuginea that may aid in stabilizing the cylinder. Zuckerman et al. described a method in which they create a neotunica with polytetrafluoroethylene (GORE-TEX®, Gore Medical, Flagstaff, AZ, USA), which covers the entire prosthesis. The proximal prosthesis is subsequently anchored to the inferior pubic ramus with 3-0 GORE-TEX® suture (11). The authors assert that the neotunica aids in alignment of the neophallus and helps secure the proximal cylinder. They also claim that GORE-TEX® provides adequate stretch for the cylinder when inflated (11). Other groups have reported that encasing the entire cylinder in graft material may result in higher rates of mechanical failure and cylinder aneurysm due to excess constraint on the cylinder during inflation (14,25).
In another technique, Falcone et al. describe using a silver-coated polyethylene terephthalate (Dacron®) graft to fashion a “sock and cap” that encases only the proximal and distal ends of the cylinder, leaving the body of the cylinder exposed. In this approach, the cylinder tubing is incorporated into the Dacron® sock, which further stabilizes the implant. It is proposed that the Dacron® cap minimizes cylinder mobility and reduces the risk of distal erosion. The Dacron® sock is then anchored to the pubis using polyester suture (14).
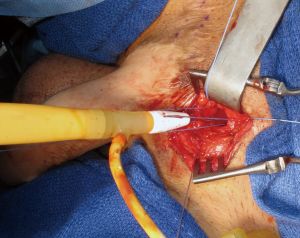
Without adequate proximal anchoring, repeated impact on the distal cylinder can result in proximal migration. This displacement may result in tethering of the cylinder to the nociceptor-containing periosteum, resulting in pain. To avoid these complications, we first perform a corticotomy in the pubic ramus in the shape of an inverted cone with a DePuy Synthes (Johnson & Johnson, New Brunswick, NJ, USA) bone drill. The corticotomy is drilled at a size to match the proximal aspect of the cylinder and/or rear tip (13,28). The use of Mitek (Mitek Surgical Products, Norwood, MA, USA) bone anchors can aid in fixation though are not usually necessary. The proximal rear tip is then seated in the corticotomy and secured to the pubis with permanent sutures (Figure 1). Narrow pilot holes targeted towards the main corticotomy defect can aid in the passage of needles through the bone, which can sometimes be a challenging endeavor. This connection can be further buttressed using a permanent mesh strip, ensuring the proximal cylinder is well-supported and seated in the appropriate position (13).
An alternative technique involves dissection under the pubic symphysis and placing multiple permanent sutures through the periosteum of the pubis and through the proximal silicone block of the implant without a rear tip. This fixation can be buttressed by a suture-wrap of permanent suture around the proximal aspect of the device, similar to a “drain stitch”.
Unfortunately, studies of outcomes of IPP insertion in the transgender male are generally in the form of single center series of a particular technique. The lack of comparison studies between techniques limits meaningful conclusions on optimal method of implantation.
Insertion of the reservoir and pump
When placing a three-piece IPP, the reservoir can be placed in the space of Retzius by blind puncture through the transversalis fascia. Some authors favor an ectopic placement given that most transgender males have undergone an abdominal hysterectomy (27). Reservoir placement may also be influenced by the presence of an incompletely empty bladder due to catheterization issues mentioned previously. To avoid potential complications from reservoir placement, some experts have recommended using an AMS Ambicor® (Boston Scientific, Inc., Watertown, MA, USA) 2-piece model in transgender men (10,31). However, care should be taken if a suture-based approach is planned since the proximal aspect of the cylinder is fluid filled in the AMS Ambicor® device. High submuscular or counter-incisions provide straightforward alternatives to conventional prevesical reservoir placement.
Pump placement can be challenging given space limitations in the neo-scrotum. Ultimately, a counter-incision may be required to properly position the pump. If two testicular prosthesis were previously inserted, it is common to remove one in exchange for the pump. The potential that both testicular prostheses will need to be removed to accommodate the pump should be discussed with the patient pre-operatively.
Post-operative care
For dressing care, we typically create a “penis palace” made from the cylindrical sides of a normal saline bottle coated with adhesive foam (Figure 2). Kerlix gauze provides circumferential pressure on the phallus and scrotum to prevent hematoma formation. Care should be made not to strangulate the phallus and limit venous outflow. Edema in the neophallus, if left in a dependent position, could provide enough pressure to malposition the tip of the cylinder proximally. If concerned, the suture used to pass the cylinder into the correct position during surgery can be left in place on tension, fixed to the dressing, overnight. During the recovery period, some surgeons opt to keep the prosthesis partially inflated for up to one week to reduce the risk of device rotation, cylinder migration, and hematoma formation (14,25). Alternatively, other surgeons do not utilize this method (10).
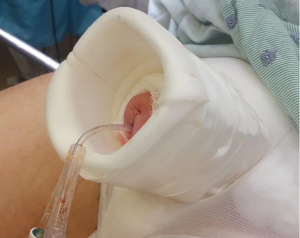
Patients are typically kept in the hospital overnight. If a urethral catheter is left, it is removed on the first post-operative day. If prolonged catheter placement is necessary, we consider placing a suprapubic tube to avoid trauma to the neourethra. Patients are taught to cycle and use the IPP 6–8 weeks after surgery.
Post-operative complications
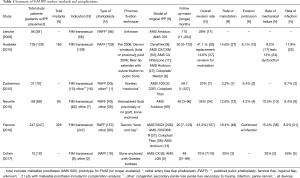
Despite continuing advancements and adjustments in technique, morbidity associated with IPP placement in transgender men exceeds IPP placement in cis-men. The rate of IPP revision or replacement in transgender men is 23–70% (9-14,25). In these complex cases, device failure is observed at earlier time intervals and risk of infection during reoperation is higher. The leading causes of reoperation include mechanical failure, cylinder erosion, device malrotation, and infection (Table 1).
Full table
Approaches for IPP revision in cases of malrotation and erosion are not well described. Using the bone anchoring technique, we have observed rare cases of sexual encounters that cause tension on the neophallus pulling the cylinders out of place. Anecdotally, some of these experiences come from manual manipulation rather than penetrative intercourse as the device is “pulled” out of the corticotomy. We have found success in repeating the initial technique utilizing a Goretex sheet to further stabilize the IPP (13). We routinely counsel patients to avoid pulling the device outwardly to reduce dislodgement.
In the era of antibiotic-impregnated IPP, infection rates in cis-gender men are cited at about 1.1% (32). Comparatively, large retrospective series of transgender male IPP recipients have cited infection rates of 8–12% (9,10,14). It is hypothesized that extensive scar tissue and subpar vascularization of the neophallus may contribute to this higher observed infection rate (9). No studies to date have identified unique measures to minimize infection in this population, so the same strategies that are utilized in cis-gender men are also adapted to this group. Specifically, we meticulously trim all visible hair, perform a multiple-step skin preparation with chlorhexidine, alcohol and iodine solutions, minimize contact of the implant with skin, and curtail operating room traffic. Regarding perioperative antibiotic regimen, our team follows current AUA guidelines for implanted prostheses, in which an aminoglycoside plus vancomycin is administered for 24 hours. Our patients are also discharged with antibiotics for one week post-operatively, though the efficacy of this practice remains controversial (33,34).
The long-term reliability and longevity of penile prostheses in transgender individuals has not been well studied. With the heterogeneity in device type and surgical method even within the same series, survival analyses are limited (Table 2). Falcone et al. performed Kaplan-Meier survival analysis in their series, which demonstrated an overall device life expectancy of 78% at 5 years (14). In contrast, though IPP longevity in the cis-male varies by device, overall and mechanical survival rates at 5 years are approximately 87–93% (35,36). Experts postulate that these differences in device survival may be attributed to anatomical differences and possibly increased sexual activity in the transgender population resulting in earlier mechanical failure (10,14).

Full table
Given the exceptionally high rates of complications, further development of trans-specific penile prostheses and refinement of techniques are needed.
Patient satisfaction
Ultimately, the goal of IPP implantation in transgender men is to successfully achieve the ability to perform penetrative intercourse. Preoperative counseling and expectation management are critically important to minimize post-operative dissatisfaction. Patients should be cautioned that IPP implantation does not affect erogenous sensation or neophallus length (37). Additionally, transgender males may feel that the device does not allow for a rigid “glans” as dissection often spares very distal penis to prevent erosion. Despite the high complication rates and need for reoperation, studies demonstrate that most individuals are satisfied with their outcomes. In a survey by Falcone et al., of 104 patients who underwent phalloplasty with IPP, 77% were able to engage in penetrative intercourse, 61% were able to achieve orgasm, and 88% were satisfied with the overall outcome of their phalloplasty (14). Zuckerman et al. found that 81% of patients in their series had a functional IPP and were sexually active at a mean 59 month follow up (11). There is no validated patient-reported outcome measure (PROM) for transgender men, so quality of life data must be interpreted with caution.
Testicular prosthesisOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
Testicular prosthesis after transgender scrotoplasty
Once an adequate neo-scrotum is created, patients may opt to pursue testicular implants. Most surgeons delay testicular implants 6–12 months after scrotoplasty. Some authors have advocated for the use of scrotal tissue expanders several months preoperatively, though tissue expansion adds an additional step to this already complex reconstructive endeavor (38). Testicular prostheses are available in a variety of sizes to accommodate an individual patient. Most transgender men opt for medium or large sizes.
Small incisions are made bilaterally in the lateral neo-scrotum, the former labia majora. Next, a pouch is developed to accommodate the prosthetic. To prevent the skin from excess stretching, various techniques to create the pouch have been described. These include using a hemostat or ringed forceps to develop and expand the potential space or inserting a Foley catheter via the skin incision and inflating the balloon (39). By minimizing tension of the skin incision, the risk of prosthetic extrusion is decreased. Especially in patients with atrophied neo-scrotal tissue or significant scarring, care should be taken to place the implant in a plane deep to fatty tissue.
The implant is then inserted into the pouch after being soaked in an antibiotic solution. Present on some implants exists a suture loop meant to aid in fixation of the implant to a dependent portion of the neo-scrotum. Most commonly, surgeons invert the neo-scrotal skin and secure the prosthesis with a suture. Care must be taken to avoid skin penetration (i.e., button-holing), which increases the risk of infection and implant extrusion (40). Some surgeons have abandoned the practice of suture fixation, allowing the prosthesis to move naturally within the neo-scrotum. In these cases, post-operative adhesions may prevent implant migration (21,41).
If a patient is simultaneously undergoing IPP placement, an option is to place only one testicular implant. The pump will act as the second implant in the contralateral neo-scrotum. In some cases, the pump alone may be placed either by patient preference or due to neo-scrotum size limitation.
Complications
While testicular prostheses are an important aspect of achieving desirable cosmesis, complications occur frequently. Commonly reported issues associated with implants in cis-gender men include graft extrusion, graft dislocation, scrotal contraction, pain, hematoma, and infection. In large series of cis-gender males, however, reoperation rates and risk of extrusion is exceedingly low (42). Although literature reviewing outcomes of testicular implants in transgender men is comparatively deficient, one series outlined complication rates in this population (43). In this series of 70 transgender men, approximately 50% experienced dislocation of either one or both testicular prostheses, most of which required surgical correction. About 30% had loss of one or both testicular implants as a result of various complications, including infection, wound dehiscence, and obstruction of urinary flow by external pressure on the neourethra. Malposition of the testicular prosthesis is commonly in a posterior plane (in the perineum between the thighs), which can affect sitting or cycling.
Future directionsOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
Although many surgical advances have been made in recent years within the realm of prosthetics for transgender men, this field remains a fertile ground for innovation. One area of development focuses on creating pumpless and reservoir-free devices to avoid the morbidity associated with placement of these components in reconstructed tissue. In this realm, one group has developed a magnetically-induced non-hydraulic shape memory alloy based penile prosthesis (44). Though this pump-free device has been tested in cadaveric studies, the exact durability and mechanism of proximal fixation have yet to be determined.
An emphasis has also been placed on development of a transgender-specific penile prosthesis. A European company, Zephyr Surgical Implants, has introduced the ZSI 475 FtM, a single cylinder three-piece inflatable prosthetic engineered for the neophallus (45). This device features a proximal flat end to allow for easier securement to the pubic bone, a wider single cylinder, a distal glans-shaped end, and a testicle-shaped pump. Though preliminary results have been promising, longer-term studies are needed to determine the comparative utility of this device. Finally, with cadaveric penile transplantation emerging as an option for patients with genital loss, this may conceivably serve as a future option for transgender men (46,47). With greater recognition of the needs of transgender patients, we are hopeful that further developments of trans-specific devices are on the horizon.
AcknowledgmentsOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
None.
FootnoteOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Introduction and background
- Penile prosthesis
- Testicular prosthesis
- Future directions
- Acknowledgments
- Footnote
- References
- Arcelus J, Bouman WP, Van Den Noortgate W, et al. Systemic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry 2015;30:807-15. [Crossref] [PubMed]
- Meerwijk EL, Sevelius JM. Transgender Population Size in the United States: a Meta-Regression of Population-Based Probability Samples. Am J Public Health 2017;107:e1-8. [Crossref] [PubMed]
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? The Williams Institute, 2016.
- Bogoraz NA. On complete plastic reconstruction of a penis sufficient for coitus. Sov Surg 1936;8:303-9.
- Bluebond-Langner R, Redett RJ. Phalloplasty in Complete Aphallia and Ambiguous Genitalia. Semin Plast Surg 2011;25:196-205. [Crossref] [PubMed]
- Hage JJ, Bout CA, Bloem JJ, et al. Phalloplasty in female-to-male transsexuals: What do our patients ask for? Ann Plast Surg 1993;30:323-6. [Crossref] [PubMed]
- Hage JJ, Bloem JJ, Bouman FG. Obtaining rigidity in the neophallus of female-to-male transsexuals: A review of the literature. Ann Plast Surg 1993;30:327-33. [Crossref] [PubMed]
- Puckett CL, Montie JE. Construction of male genitalia in the transsexual, using a tubed groin flap for the penis and a hydraulic inflation device. Plast Reconstr Surg 1978;61:523-30. [Crossref] [PubMed]
- Hoebeke PB, Decaestecker K, Beysens M, et al. Erectile implants in female-to-male transsexuals: our experience in 129 patients. Eur Urol 2010;57:334-40. [Crossref] [PubMed]
- Neuville P, Morel-Journel N, Maucourt-Boulch D, et al. Surgical Outcomes of Erectile Implants After Phalloplasty: Retrospective Analysis of 95 Procedures. J Sex Med 2016;13:1758-64. [Crossref] [PubMed]
- Zuckerman JM, Smentkowski K, Gilbert D, et al. Penile Prosthesis Implantation in Patients with a History of Total Phallic Construction. J Sex Med 2015;12:2485-91. [Crossref] [PubMed]
- Leriche A., Timsit MO, Morel-Journel N, et al. Long-term outcome of forearm flee-flap phalloplasty in the treatment of transsexualism. BJU Int 2008;101:1297-300. [Crossref] [PubMed]
- Cohen AJ, Bhanvadia RR, Pariser JJ, et al. Novel Technique for Proximal Bone Anchoring of Penile Prosthesis After Radial Forearm Free Flap Neophallus. Urology 2017;105:2-5. [Crossref] [PubMed]
- Falcone M, Garaffa G, Gillo A, et al. Outcomes of inflatable penile prosthesis insertion in 247 patients completing female to male gender reassignment surgery. BJU Int 2018;121:139-44. [Crossref] [PubMed]
- Trombetta C, Liguori G, Bertolotto M. Management of Gender Dysphoria: A Multidisciplinary Approach. Springer, Milano, 2015.
- Gilbert DA, Winslow BH, Gilbert DM, et al. Transsexual surgery in the genetic female. Clin Plast Surg 1988;15:471-87. [PubMed]
- DiGeronimo EM. Scrotal reconstruction utilizing a unilateral adductor minimus myocutaneous flap. Plast Reconstr Surg 1982;70:749-51. [Crossref] [PubMed]
- Hage JJ, Bouman FG, Bloem JJ. Constructing a scrotum in female-to-male transsexuals. Plast Reconstr Surg 1993;91:914-21. [Crossref] [PubMed]
- Selvaggi G, Hoebecke P, Ceulemans P, et al. Scrotal reconstruction in Female-to-Male Transsexuals: the novel scrotoplasty. Plast Reconstr Surg 2009;123:1710-8. [Crossref] [PubMed]
- Sengezer M, Sadove RC. Scrotal construction by expansion of labia majora in biological female transsexuals. Ann Plast Surg 1993;31:372-6. [Crossref] [PubMed]
- Yossepowitch O, Aviv D, Wainchwaig L, et al. Testicular Prosthees for Testis Cancer Survivors: Patient Perspectives and Predictors of Long-Term Satisfaction. J Urol 2011;186:2249-52. [Crossref] [PubMed]
- Bodiwala D, Summerton DJ, Terry TR. Testicular Prostheses: Development and Modern Usage. Ann R Coll Surg Engl 2007;89:349-53. [Crossref] [PubMed]
- Morrison SD, Massie JP, Dellon AL. Genital Sensibility in the Neophallus: Getting a Sense of the Current Literature and Techniques. J Reconstr Microsurg 2019;35:129-37. [Crossref] [PubMed]
- Selvaggi G, Monstrey S, Ceulemans P, et al. Genital sensitivity after sex reassignment surgery in transsexual patients. Ann Plast Surg 2007;58:427-33. [Crossref] [PubMed]
- Hoebeke P, de Cuypere G, Ceulemans P, et al. Obtaining rigidity in total phalloplasty: experience with 35 patients. J Urol 2003;169:221-3. [Crossref] [PubMed]
- Levine LA, Zachary LS, Gottlieb LJ. Prosthesis placement after total phallic reconstruction. J Urol 1993;149:593-8. [Crossref] [PubMed]
- Levine LA, Becher E, Bella A, et al. Penile Prosthesis Surgery: Current Recommendations from the International Consultation on Sexual Medicine. J Sex Med 2016;13:489-518. [Crossref] [PubMed]
- Large MC, Gottlieb LJ, Wille MA, et al. Novel technique for proximal anchoring of penile prostheses in female-to-male transsexual. Urology 2009;74:419-21. [Crossref] [PubMed]
- Smith CP, Kraus SR, Boone TB. Management of impending penile prosthesis erosion with a polytetrafluoroethylene distal wind sock graft. J Urol 1998;160:2037-40. [Crossref] [PubMed]
- Carson CC, Noh CH. Distal penile prosthesis extrusion: treatment with distal corporoplasty or Gortex windsock reinforcement. Int J Impot Res 2002;14:81-4. [Crossref] [PubMed]
- Abdelsayed GA, Levine LA. Ambicor 2-Piece Inflatable Penile Prosthesis: Who and How? J Sex Med 2018;15:410-5. [Crossref] [PubMed]
- Carson CC, Mulcahy JJ, Harsch M. Long-term infection outcomes after original antibiotic-impregnated inflatable penile prosthesis implants: up to 7.7 of follow-up. J Urol 2011;185:614-8. [Crossref] [PubMed]
- Wolf JS Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008;179:1379-90. [Crossref] [PubMed]
- Adamsky MA, Boysen WR, Cohen AJ, et al. Evaluating the Role of Postoperative Oral Antibiotic Administration in Artificial Urinary Sphincter and Inflatable Penile Prosthesis Explantation: A Nationwide Study. Urology 2018;111:92-8. [Crossref] [PubMed]
- Chung E, Solomon M, DeYoung L, et al. Comparison between AMS 700™ CX and Coloplast™ Titan inflatable penile prosthesis for Peyronie's disease treatment and remodeling: clinical outcomes and patient satisfaction. J Sex Med 2013;10:2855-60. [Crossref] [PubMed]
- Kim DS, Yang KM, Chung HJ, et al. AMS 700CX/CXM inflatable penile prosthesis has high mechanical reliability at long-term follow-up. J Sex Med 2010;7:2602-7. [Crossref] [PubMed]
- Garcia MM, Christopher NA, De Luca F, et al. Overall satisfaction, sexual function, and the durability of neophallus dimensions following staged female to male genital gender confirming surgery: The Institute of Urology, London U.K. experience. Transl Androl Urol 2014;3:156-62. [PubMed]
- Small MP, Becker H. Use of tissue expanders in genitourinary reconstructive surgery for transsexuals. In: Proceedings of the eleventh Harry Benjamin International Gender Dysphoria Association Symposium. Cleveland, OH, 1989.
- Chadha A, Saraiya H. Scrotal reconstruction using Foley catheters as tissue expanders. Ann Plast Surg 1991;26:291-2. [Crossref] [PubMed]
- Donati-Bourne J, Deb A, Mathias SJ, et al. Complete Expulsion of Testicular Prosthesis via the Scrotum: A Case-Based Review of the Preventive Surgical Strategies. Case Rep Urol 2015;2015:434951. [Crossref] [PubMed]
- Zilberman D, Winkler H, Kleinmann N, et al. Testicular prosthesis insertion following testicular loss or atrophy during early childhood--technical aspects and evaluation of patient satisfaction. J Pediatr Urol 2007;3:461-5. [Crossref] [PubMed]
- Marshall S. Potential problems with testicular prostheses. Urology 1986;28:388-90. [Crossref] [PubMed]
- Hage JJ, van Turnhout AA. Long-term outcome of metaidoioplasty in 70 female-to-male transsexuals. Ann Plast Surg 2006;57:312-6. [Crossref] [PubMed]
- Le B, McVary K, Colombo A. The performance characteristics of a shape memory penile prosthesis in human cadavers. J Urol 2018;199:e393. [Crossref]
- Neuville P, Terrier JE, Paparel P, et al. Preliminary study of a specific penile prosthesis conceived for phalloplasty, the ZSI® 475 FtM. J Sex Med 2018;15:S197. [Crossref]
- Szafran AA, Redett R, Burnett AL. Penile transplantation: The US experience and institutional program set-up. Transl Androl Urol 2018;7:639-45. [Crossref] [PubMed]
- Selvaggi G, Wesslen E, Elander A, et al. En Bloc Surgical Dissection for Penile Transplantation for Trans-Men: A Cadaveric Study. Biomed Res Int 2018;2018:6754030. [Crossref] [PubMed]