
Sertoli cell only syndrome induced by a varicocele
Introduction
Approximately 15% of couples in North America present for a fertility evaluation after being unsuccessful at achieving a pregnancy for one year with unprotected intercourse. Of the couples struggling to conceive, male factor is solely responsible for 20% of these cases, while it is a contributory factor in conjunction with female infertility factors in an additional 40%, resulting in a 60% incidence of male factor involvement in infertile couples (1). One percent of men in the general population are azoospermic and approximately 15% of men presenting for infertility evaluations are found to be azoospermic (2). Azoospermia is defined as the absence of spermatozoa in the ejaculate following centrifugation and subsequent microscopy of the specimen on two separate semen analyses (3), whereas non-obstructive azoospermia (NOA) is azoospermia due to impairment of testicular sperm production. Sertoli cell only syndrome (SCOS) is the most severe form of NOA with surgical sperm retrieval rates with microdissection testicular sperm extraction (microTESE) of 22.5–41%, significantly lower than microTESE sperm retrieval rates with other histological patterns such as hypospermatogenesis (73–100%), late maturation arrest (27–86%), or early maturation arrest (27–40%) (4,5).
Varicoceles are abnormally dilated scrotal veins of the pampiniform plexus, which are found in approximately 15% of men in the general population and in 40% of men presenting for infertility evaluations, making varicocele the most common diagnosis made in infertile men (6). As one percent of men in the general population are azoospermic and 15% of men presenting for infertility are azoospermic, there is a fair amount of overlap in azoospermic men with varicoceles (1,6). There are a number of hypotheses of the mechanisms by which varicoceles may adversely impact spermatogenesis and testicular function which may potentially lead to azoospermia. The majority of the data indicate that varicoceles impact testicular function by increasing intratesticular temperatures due to interruption of counter-current heat exchange in the pampiniform plexus with opposing flows in a central arterial system (7). The potential mechanisms of cellular damage from varicoceles that have been proposed include sperm DNA fragmentation, apoptosis, increasing reactive oxygen species through oxidative stress, intracellular ionic and metabolic changes, and predisposition to sperm aneuploidy (8,9). Men with varicoceles demonstrate higher mean tubular apoptotic indices than controls (10).
Case presentation
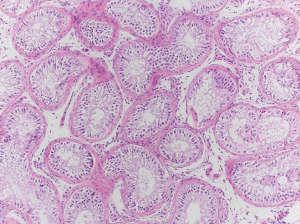
After Institutional Review Board exemption was obtained, a chart review of a patient’s case was performed to collect the data for this report. A 34-year-old man with a 35-year-old wife presented for fertility evaluation after being unsuccessful at achieving a pregnancy for 14 months. They have a three-and-a-half-year-old son and although it took six months to conceive at that time, they conceived spontaneously. They underwent fertility evaluation at that time as well. His semen analysis report from that time demonstrated a semen volume of 2.1 mL, a sperm concentration of 21 million sperm per mL of semen, total motility of 44%, forward progressive motility of 26%, and normal morphology by Kruger strict criteria of 3%. They ultimately conceived spontaneously with timed intercourse at that time, over four years ago. Reproductive endocrinology evaluation ruled out female fertility factors. Current assessment of the male partner revealed no significant lifestyle factors or medications that would impact spermatogenesis, the man had no significant heat exposure and was not using any androgenic products, was not a smoker and had minimal alcohol intake. His testicular volumes were measured to be 9 cc bilaterally by Prader orchidometer, he had a follicle stimulating hormone (FSH) of 22.5 mIU/mL, luteinizing hormone (LH) of 5.1 IU/L, prolactin of 7.7 ng/mL, testosterone of 311 ng/dL, and estradiol of 29 pg/mL. His karyotype revealed a normal 46, XY karyotype and his Y chromosome microdeletion assay revealed no microdeletions. He had a grade three left varicocele which was visible and palpable on physical examination, and two semen analyses revealing azoospermia with no sperm in the centrifuged, concentrated pellets. He was counseled and underwent left subinguinal microsurgical varicocele repair and remained azoospermic at three months and six months post-operatively. His physical examination at both those time intervals revealed no varicocele by visualization or palpation with Valsalva maneuver. He was counseled and elected to proceed with microTESE. He underwent bilateral microTESE with no sperm identified in any specimens. Testicular biopsy at the time of microTESE sent for permanent section pathology ultimately revealed a SCOS pattern (Figure 1).
Discussion
Varicoceles are well known to induce testicular injury and impact spermatogenesis. There is a suggested role for varicocele repair in men with NOA with palpable varicoceles. Previous studies by Matthews et al., Kim et al., and Saleh et al. have shown return of sperm to the ejaculate in men with NOA following varicocele repair. Return of sperm to the ejaculate is reported in approximately 10% to 50% of men with NOA post-varicocele repair, the majority of which show more favorable outcomes associated with testicular histology of hypospermatogenesis or late maturation arrest, as opposed to less favorable outcomes after repair in men with SCOS or early maturation arrest (11-13). Other studies revealed that varicocele repair in men with NOA improve sperm retrieval rates at the time of microTESE and improves in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) outcomes. Another study, which was a retrospective review performed by Haydardedeoglu et al., revealed a sperm retrieval rate of 60.8% in men who remained azoospermic after varicocele repair versus a sperm retrieval rate of 38.5% in NOA men who did not have the varicocele repaired. The varicocele repair group also had a significantly higher clinical pregnancy rate and live birth rate with IVF/ICSI than the men who did not undergo repair, 74.2% versus 52.3% and 64.5% versus 41.5%, respectively (14). Another study by Inci et al. assessed couples who underwent IVF/ICSI when men underwent varicocele repair versus men who left their varicoceles untreated. The sperm retrieval rate with microTESE was significantly higher in the men who previously had their varicoceles repaired, 53% versus 30%. However, there was no difference in fertilization rate, rate of high quality embryos, or mean number of transferred embryos. The clinical pregnancy rate was significantly higher in the varicocele treated group versus the untreated group, at 31% versus 22%, respectively (15). Other studies, including a prospective study by Aboutaleb et al. and an observational study by Zampieri et al., have shown similar results of improved sperm retrieval rates, fertilization rates, pregnancy rates, and live birth rates with microTESE/IVF/ICSI after varicocele repair versus leaving varicoceles intact (16,17). Another previous study by Ustuner et al. suggested that testicular histology has the potential to improve after varicocele repair in men with NOA. Testicular biopsy was performed in men at the time of varicocele repair and again at the time of microTESE in men who remained azoospermic after varicocele repair. Fourteen men were classified as SCOS from the biopsies at the time of varicocele repair and were reclassified as focal spermatogenesis in two of them and late maturation arrest in three of them from the biopsies at the time of microTESE (18). All of these studies suggest that varicocele repair has the potential of improving testicular function and physiology.
To our knowledge, the current case study is the first reported which reveals that there is a potential for a fertile man to have a high-grade varicocele induce testicular injury devastating enough to result in SCOS with no sperm retrievable at the time of microTESE. Although he had a unilateral varicocele, there has been evidence in animal studies, such as the one conducted by Ozturk et al., that a unilateral varicocele can induce bilateral testicular histopathologic injury (19). Human studies, such as the one performed by Benoff et al., have also revealed increased levels of apoptosis bilaterally in the testes of men with unilateral varicoceles (20). A previous study by Gat et al. described 13 patients with bilateral varicoceles and SCOS. Azoospermia was found on testicular biopsy in most of these men after definitive therapy (21). However, the unique aspect of this current case report is the longitudinal nature of it in this single patient, showing clear progression from being a fertile male capable of conceiving and achieving a live birth with his wife spontaneously, to NOA with Sertoli cell only histology. This case stands out as the patient was identified as being fertile in the past and progressing to the severest form of secondary infertility with a varicocele as the only identifiable etiology.
Although it is true that varicoceles are very common, identified in approximately 15% of men in the general population and 20% of men presenting for a fertility evaluation, in this scenario, it is likely that Sertoli cell only is due to the varicocele. Unfortunately, we do not have longitudinal data with semen analyses over time in men who ultimately are found to have Sertoli cell only pattern in the testes, so it is unknown if the men with NOA who do not have an identifiable inciting etiology for this pathology such as chemotherapy have always been azoospermic or if they develop azoospermia. In this particular case of a man with a history of reasonable semen parameters at the time he was able to achieve a pregnancy with his wife in the past who progressed to NOA with the only identifiable risk factor being a varicocele, the most likely scenario is progression of testicular injury due to the varicocele to the end point of SCOS. If he had always been azoospermic with a biopsy revealing Sertoli cell only at the time of microTESE, this association would be much less likely. The sequence of events in this patient supports the conclusion.
This case indicates that a man with secondary infertility with the previous ability to father a child with timed intercourse had progression to NOA with SCOS due to a varicocele without other identifiable etiologies for infertility. This suggests that a high-grade varicocele can induce sufficient testicular injury to result in the most severe testicular histological architecture associated with NOA.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from the patient in surgical consents to use surgical/pathologic imaging and to present case.
References
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6. [Crossref] [PubMed]
- Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol 1989;142:62-5. [Crossref] [PubMed]
- Avila-Flores R, Medellin RA. Ecological, taxonomic, and physiological correlates of cave use by mexican bats. J Mammal 2004;85:675-87. [Crossref]
- Bernie AM, Shah K, Halpern JA, et al. Outcomes of microdissection testicular sperm extraction in men with nonobstructive azoospermia due to maturation arrest. Fertil Steril 2015;104:569-73.e1. [Crossref] [PubMed]
- Caroppo E, Colpi EM, Gazzano G, et al. Testicular histology may predict the successful sperm retrieval in patients with non-obstructive azoospermia undergoing conventional TESE: a diagnostic accuracy study. J Assist Reprod Genet 2017;34:149-54. [Crossref] [PubMed]
- Nagler HM, Luntz RK, Martinis FG. Varicocele. In: Lipshultz LI, Howards SS. editors. Infertility in the male. St. Louis, MO. Mosby Year Book, 1997:336-59.
- Zorgniotti AW, Macleod J. Studies in temperature, human semen quality, and varicocele. Fertil Steril 1973;24:854-63. [Crossref] [PubMed]
- Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod 2006;21:986-93. [Crossref] [PubMed]
- Baccetti BM, Bruni E, Capitani S, et al. Studies on varicocele III: ultrastructural sperm evaluation and 18, X and Y aneuploidies. J Androl 2006;27:94-101. [Crossref] [PubMed]
- Hassan A, el-Nashar EM, Mostafa T. Programmed cell death in varicocele-bearing testes. Andrologia 2009;41:39-45. [Crossref] [PubMed]
- Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril 1998;70:71-5. [Crossref] [PubMed]
- Kim ED, Leibman BB, Grinblat DM, et al. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol 1999;162:737-40. [Crossref] [PubMed]
- Saleh R, Agarwal A, Farouk H. A rational approach to the management of varicocele-associated nonobstructive azoospermia. Fertil Steril 2011;95:489-90. [Crossref] [PubMed]
- Haydardedeoglu B, Turunc T, Kilicdag EB, et al. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: a retrospective pilot study. Urology 2010;75:83-6. [Crossref] [PubMed]
- Inci K, Hascicek M, Kara O, et al. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol 2009;182:1500-5. [Crossref] [PubMed]
- Aboutaleb HA, Elsherif EA, Omar MK, et al. Testicular Biopsy Histopathology as an Indicator of Successful Restoration of Spermatogenesis after Varicocelectomy in Non-obstructive Azoospermia. World J Mens Health 2014;32:43-9. [Crossref] [PubMed]
- Zampieri N, Bosaro L, Costantini C, et al. Relationship between testicular sperm extraction and varicocelectomy in patients with varicocele and nonobstructive azoospermia. Urology 2013;82:74-7. [Crossref] [PubMed]
- Ustuner M, Yilmaz H, Yavuz U, et al. Varicocele Repair Improves Testicular Histology in Men with Nonobstructive Azoospermia. Biomed Res Int 2015;2015:709452. [Crossref] [PubMed]
- Ozturk MI, Koca O, Keles MO, et al. The impact of unilateral experimental rat varicocele model on testicular histopathology, Leydig cell counts, and intratesticular testosterone levels of both testes. Urol J 2013;10:973-80. [PubMed]
- Benoff SH, Millan C, Hurley IR, et al. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum Reprod 2004;19:616-27. [Crossref] [PubMed]
- Gat Y, Gornish M, Perlow A, et al. Azoospermia and Sertoli-cell-only syndrome: hypoxia in the sperm production site due to impairment in venous drainage of male reproductive system. Andrologia 2010;42:314-21. [Crossref] [PubMed]


