Should perioperative anticoagulation be an integral part of the priapism shunting procedure?
We propose that post-shunting recurrence of ischemic priapism is a postoperative thromboembolic complication, similar to cases of postoperative thrombosis of veins (femoral or popliteal) or arteries (cerebral or coronary). Therefore, perioperative anticoagulation should be given to men undergoing these shunting procedures to prevent this complication.
Two illustrative cases are presented herein.
Case 1
The patient is a 22-year-old man with a history of schizophrenia, controlled with oral medications (olanzapine and valproic acid). Over the past two years, he had eight episodes of ischemic priapism, lasting between 4-14 hours, which all resolved spontaneously.
The patient presented again to a local hospital with a chief complaint of painful erection for three days. The patient was immediately transferred to our center. A thorough history yielded only recent (and only intermittent) use of olanzapine as a possible causative factor for the ischemic priapism. He complained of severe, dull pain in his penis. On physical examination, his penis was rigid and tender. There were no signs of infection. His WBC count was normal.
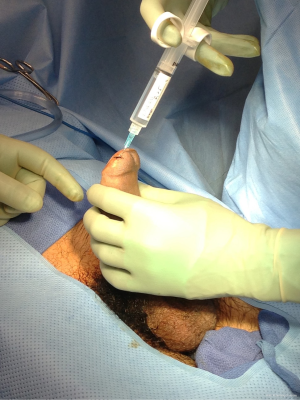
He was taken to the operating room, as he declined bedside procedure in the emergency department. On exam, the tip of the rigid corporal body was easily palpable at a shallow depth from the surface of the glans. A marking pen was used to make two vertical incision lines about 1 cm long, 0.5 cm lateral to urethral meatus. Local anesthetic (0.25% bupivicaine) was injected into the subepithelial layer (not the underlying spongy tissue) of the glans overlying the planned, T-shunt sites (Figure 1). Bilateral T-shunts were then performed using a 10-blade scalpel, in quick succession. Bilateral corporal tunneling was performed using 22-Fr. straight urethral sounds as previously described (1-3). With the sound oriented slightly laterally, the sound was passed gently to the crura without injury to the urethra. After the sound was removed, there was immediate drainage, of thick dark viscous blood. The penis was milked until blood draining forth from the shunt sites turned into a bright-red color. The T-shunt sites were closed with running-locking 4-0 chromic sutures. Care was taken to place each suture shallowly within glans tissue, so as to minimize incorporation of deeper glans tissue at the shunt site. The penis was moderately edematous, but remained non-erect throughout a 10-minute observation period and through the end of the surgery. A Foley catheter was placed which drained clear yellow urine. Following transfer to the recovery room, the patient was discharged to the ward for observation.
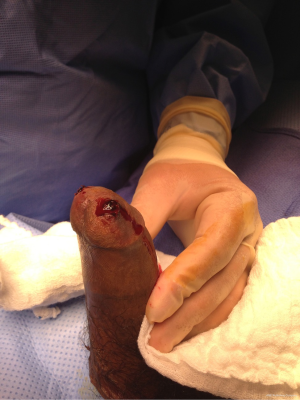
The patient was found to have a partially erect phallus until the evening. However, the next morning, the patient awoke with a rigid erection. Based on our exam, the patient appeared to have early recurrence of ischemic priapism. He consented to our recommendation to return to the operating room for repeat T-shunt and tunneling. We also explained to him our recommendation of perioperative anticoagulation. He was given subcutaneous heparin 5,000 units pre-operatively. In the operating room, approximately 15 hours after his previous surgery, we removed the sutures on the glans. Upon doing so, a small clot immediately presented itself below the suture line on each side (Figure 2). With repeat T-shunt and tunneling, there was drainage of very dark blood that was less viscous than what was drained at surgery 15 hours prior. There was no additional clot noted in the blood evacuated from the corporal bodies. As before, we milked the penis until the first return of fresh bright blood. We observed the penis for several minutes and noted no recurrence of priapism. We then proceeded to close the T-shunts with 4-0 chromic interrupted locking sutures. The penis remained moderately soft and not rigid through the end of the case. He was given 325 mg of aspirin and 40 mg of famotidine after recovering from anesthesia and instructed to take baby aspirin (81 mg) and famotidine (40 mg) daily for two weeks. One more subcutaneous heparin injection was given 12 hours after the initial dose.
For the next 24 hours, the penis remained partially erect, but not rigid. The patient reported no penile pain at rest, and was discharged approximately 30 hours after his second surgery. As numerous case reports have suggested that olanzapine can cause ischemic priapism, his psychiatrist was contacted before discharge. Close outpatient follow-up (within 24 hours) with his psychiatrist was arranged prior to discharge.
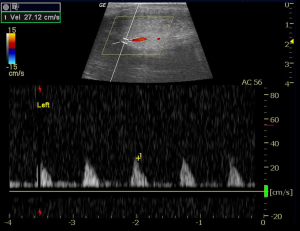
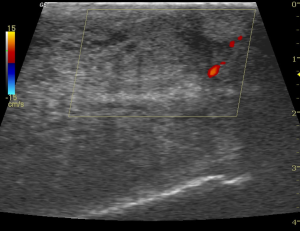
At follow-up 2-week later, the patient reported no recurrence of painful erection since discharge. He confirms that he continues to take aspirin 81 mg daily. He also confirms that he awakens each morning with a good erection (painless, about 90% rigidity, sufficient for penetration), and that these erections detumescence spontaneously. Erections recur intermittently during the day and evening. On exam, no gross swelling or ecchymosis was visible. His penis was fully engorged but soft and the mid-shaft could be easily compressed. Color duplex penile ultrasound was performed. Arterial flow was evident in each cavernous artery (Figure 3) and the glans-cavernosum shunt was patent with detectable flow (Figure 4).
Case 2
The second case is a 40-year-old politician who had suffered from anxiety and insomnia for several months because of a personal financial problem. He was given trazodone for insomnia. After taking the first dose, he woke up the next morning with a rigid erection. He was well known in the community and was embarrassed to seek medical treatment. He presented to a local hospital about 48 hours later. Aspiration of old blood and injection of a diluted phenylephrine solution failed to resolve the priapism. A Winter’s shunt was performed by a local urologist. The priapism was relieved only to recur about six hours later. He was taken to the operating room and a spongiosum-cavernosum shunt was performed. The penis became soft (but swollen) after the procedure. However, persistent painful erection occurred the next morning and he was referred to our emergency room for further management. Under local anesthesia a 10-blade scalpel was used to create a T-shunt bilaterally. This was followed by bilateral tunneling with a 22 Fr. straight female sound. The priapism subsided shortly after, and the patient was discharged home.
He returned the next day, again, with a rigid painful erection. We recommended our perioperative anticoagulation therapy and started him with 325 mg aspirin and subcutaneous injection of 5,000 units of heparin. He was brought to the operating room for a repeat T-shunt procedure. When the sutures on the glans were removed, blood clots were noted at the T-shunt site. Repeated T-shunt and tunneling was performed which resulted in a partial detumescence. Manual compression of the base of the penis dislodged a few blood clots followed by non-coagulated old blood. The penis remained at partial erection but compressible ten minutes after the wound on the glans was closed with continuous locking water-tight 4-0 chromic sutures to prevent postoperative bleeding. He was given one more dose of subcutaneous 5,000 units of heparin and instructed to take baby aspirin (81 mg) daily for two weeks after discharge. His partial erection decreased gradually and the penis was flaccid after about five days. He reported fully-regained erectile function without the need for phosphodiesterase-5 inhibitors about three months later.
Comments
In ischemic priapism that does not respond to alpha-adrenergic agonists, various shunting procedures have been developed to re-establish circulation of the corpora cavernosa and prevent necrosis of erectile tissues. However, early closure of the newly created shunt, resulting in recurrent priapism, is a common complication which leads to repeated shunting procedures, longer hospital stays, increased patient sufferings, and less than favorable outcomes. In fact, early recurrent priapism is the most common emergent phone consultation to our andrology clinic. In subsequent exploration/re-shunting, blood clots at the site of shunting can always be expelled, followed by un-coagulated “crank-case oil”-like old blood. It is well documented that the old blood inside the corpora cavernosa does not clot due to the abundance of endothelium-derived anticoagulating and fibrinolytic factors within the corpora cavernosa (4). However, the newly created shunt is not lined and protected by endothelium. In fact, the shunt cuts a new wound through the collagen-rich tunica albuginea. The collagen-activated platelets and fibrin begin to form a clot within minutes to seal off the shunt in a similar fashion as a clot forming at the site of an injured blood vessel wall (5) (Figure 5).
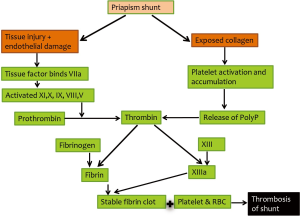
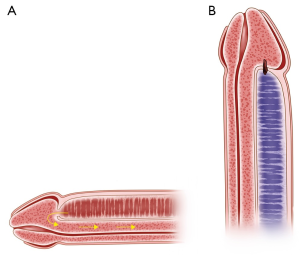
Keeping the newly created shunt patent requires a continuous high blood flow through a large caliber shunt. When all goes well, the shunt remains open for hours to days (Figure 6A) until the smooth muscles of the corpora cavernosa and helicine arteries regain normal contractile capacity (6). Once the post-ischemic high blood flow is normalized, a larger clot will form and shunt will close spontaneously. Premature closure of the shunt from inadequate size and stagnant blood flow will enhance the adherence of platelets and fibrin to collagen, eventually forming a thrombus at the site of shunting and cause early recurrence of priapism (Figure 6B). In essence, early postoperative shunt closure is a postoperative thrombotic complication, and its management/prevention should be incorporated into the future guidelines for treatment of ischemic priapism (Figure 7).

The risk of postoperative thrombo-embolic complications, such as femoral-popliteal venous thrombosis, pulmonary embolism, and cerebral and coronary arterial occlusion, is ever present, and each poses a major threat to a patient’s life. Clinical guidelines have been established (and are constantly evolving
Acknowledgements
The authors wish to acknowledge KARL STORZ Endoscopy-America, Inc. for providing the VITOM Visualization System for recording these videos.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brant WO, Garcia MM, Bella AJ, et al. T-shaped shunt and intracavernous tunneling for prolonged ischemic priapism. J Urol 2009;181:1699-705. [PubMed]
- Garcia MM, Shindel AW, Lue TF. T-shunt with or without tunnelling for prolonged ischaemic priapism. BJU Int 2008;102:1754-64. [PubMed]
- Garcia MM, Porten S, Lue TF. Commentary on refractory ischemic priapism. Transl Androl Urol 2012;1:61-5.
- Rolle L, Bazzan M, Bellina M, et al. Coagulation and fibrinolytic activity of blood from the corpus cavernosum. Arch Ital Urol Nefrol Androl 1991;63:471-3. [PubMed]
- McMichael M. New models of hemostasis. Top Companion Anim Med 2012;27:40-5. [PubMed]
- Kim NN, Kim JJ, Hypolite J, et al. Altered contractility of rabbit penile corpus cavernosum smooth muscle by hypoxia. J Urol 1996;155:772-8. [PubMed]