Contemporary lymphatic interventions for post-operative lymphatic leaks
IntroductionOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
While historically uncommon, the incidence rate of chylothorax, chylous ascites, and lymphocele is rising likely due to longer survival of cancer patients and the potential for aggressive surgery. For instance, chylous ascites complicates between 0.3–11% of major abdominal operations and lymphoceles complicate between 0.6–32% of renal transplants, urologic lymphadenectomies, and gynecologic lymphadenectomies (1-6). Manifestations may range from asymptomatic, small lymphoceles incidentally found on follow-up imaging to a recalcitrant high volume and debilitating chylous ascites. Traditionally, conservative therapy for lymphatic injuries consists of dietary modification with either a non-fat diet or total parenteral nutrition, drainage of the affected area, and supportive care. If the lymphatic leak persists, open or laparoscopic surgical approaches could be pursued at the risk of longer hospitalization and recovery, increased morbidity, and higher mortality (7-9).
Over the last two decades, lymphatic intervention has been a new frontier in interventional radiology brought to the forefront by Constantin Cope in 1999 with a prospective trial of thoracic duct embolization for chylothorax (10). Multiple subsequent techniques and applications have arisen from Cope’s initial work including successful treatment of chylous ascites, lymphoceles, plastic bronchitis, and protein losing enteropathy (11-14). Herein, an overview of lymphatic anatomy is followed by a discussion of the evolution of lymphangiography from a “lost-art” to a necessary precursor for lymphatic intervention. The current status of lymphatic intervention for traumatic chylothorax, chylous ascites, and lymphocele are then summarized followed by a suggested treatment algorithm.
Lymphatic anatomyOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
Despite dramatic advances in medical knowledge, the lymphatic circulation remains mysterious and difficult to study. Ancient Greeks were aware of the presence of lymphatic vessels, but incompletely understood their function. Autopsy and animal vivisection by multiple scholars throughout the 17th century led to a fuller understanding of lymphatic anatomy and physiology, particularly within the abdominal cavity (15). In the 19th century, the structure and function of lymph nodes and their interplay with lymphatic vessels was described. This was followed by the theory for the formation of lymph by diffusion across blood vessels by a balance of hydrostatic and oncotic pressures, the Starling equation. With the understanding that the lymphatic system functions to return fluid and nutrients from interstitial tissues to the venous system, three distinct subdivisions within the lymphatic circulation are now recognized: soft tissue/extremity, hepatic, and enteral lymphatics (15).
Functionally, the lymphatics are a unidirectional drainage system channeling 1.5–4 L of fluid back to the central circulation daily. Hepatic and enteral lymphatics produce approximately 80% of lymphatic fluid, while the upper and lower extremities give rise to the remaining 20% of daily lymph volume. While the hepatic lymphatics are rich in protein and the enteral lymphatics are rich in fat droplets, the extremity lymphatics have a low concentration of nutrients and a preponderance of lymphocytes (15). Lymphatic circulation is vital for the return of serum protein and lipids to the systemic circulation and is central to fluid balance and immune response.
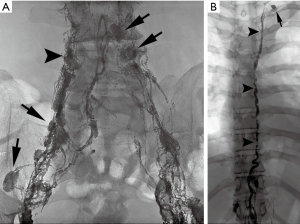
The fluid from each network courses through lacelike vessels and interspersed lymph nodes to the retroperitoneum where they coalesce to form a dilated sac-like structure, the cisterna chyli. The cisterna chyli in turn gives rise to the thoracic duct, which is the largest lymphatic vessel in the body at approximately 40 cm long and terminates at the left venous angle (Figure 1). The right head, neck, thorax, and upper extremity lymphatics drain through a right lymphatic duct. All remaining lymphatics including the hepatic, enteral, lower extremities, left upper extremities, and the left head and neck drain into the thoracic duct. This standard anatomy is present in approximately 50% of the population (15).
LymphangiographyOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
Dating back to the 1930s, contrast material was directly injected into lymph nodes for radiographic visualization (16). Kinmonth et al. described the technique of lymphangiography of the lower extremity in 1955 and Fischer and Zimmerman tested different radio-opaque contrast agents in the lymphatics, including ethiodized oil, which is used to the present day (17,18). Traditional trans-pedal lymphangiography, “the Kinmonth method”, was regularly used since 1955 for various indications including lymphoma staging, differentiation of inflammatory from neoplastic processes, evaluation of metastatic disease, and determination of chemotherapy response. Following injection of blue dye in the webspaces between the toes, surgical cutdown of the dorsum of the foot was performed to expose a lymphatic vessel, which was subsequently cannulated with a 30-gauge needle (Figure 2). An injection of approximately 5 cc/hour of ethiodized oil would then be initiated in each foot and serial spot radiographs were taken from the foot to the chest, following the progression of the contrast. However, pedal lymphangiography could take several hours to yield elegant images of lymphatic vessels and the retroperitoneal circulation. With the advent of new imaging technologies including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) diagnostic lymphangiography fell out of favor (19,20). By the 1990s, only a few medical centers performed lymphangiography as a diagnostic exam and technical proficiency of radiologists began to wane. Constantin Cope is largely credited with transitioning lymphangiography from a diagnostic test to a precursor for lymphatic interventions. Additionally, reports from other authors emerged on the therapeutic efficacy of lymphangiography in successfully treating approximately 50% of patients with various lymphatic leaks (21).
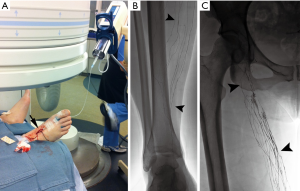
Although these initial lymphatic interventions reinvigorated interest in pedal lymphangiography, the technical difficulty, invasive nature, and time-consumption of the modality precluded wide adoption. Moreover, given the low demand for pedal lymphangiography over decades, infusion pumps necessary for the procedure became difficult to locate and service. Intranodal lymphangiography was initially described in children in 2011 and subsequently in adults in 2012, as an easier alternative with shorter procedure time (22,23). Under ultrasound guidance, a 25–27-gauge spinal needle is positioned into inguinal lymph nodes at the junction between the hilum and cortex. Subsequent slow injection (approximately 0.2–0.4 mL per minute) of ethiodized oil is then followed with fluoroscopy and spot radiographs from the inguinal lymph nodes through the pelvic lymphatic chain and into the retroperitoneal lymphatic vessels. Further, the application of intranodal lymphangiography technique with gadolinium-based contrasts have revolutionized the ability to dynamically visualize lymphatic anatomy under MRI and will allow for continued technical innovation and better understanding of lymphatic pathophysiology (24,25).
ChylothoraxOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
Historically described by Bartholin in 1651, chylothorax occurs when chylous fluid leaks into the pleural space (26). Presently, the incidence of this disorder is estimated at 1:6,000 hospital admissions (27-29). While older reports list nontraumatic causes of chylothorax, such as lymphoma, lung cancer, or tuberculosis as the most common etiologies, more recent reports note the increase in post-surgical chylothorax. For instance, chylothorax complicates 0.42% of general thoracic surgeries and up to 3.9% of esophagectomies (1,2,27-29). Patients present with symptoms related to pleural effusions including dyspnea, chest pain, fever, and/or fatigue (29). Pleural fluid that has a milky appearance with a triglyceride concentration greater than 110 mg/dL or the presence of chylomicrons on lipoprotein electrophoresis confirms the diagnosis of chylothorax (30). Traditional treatment consists of a non-fat diet or total parenteral nutrition, drainage, and supportive care often lasting for weeks or months. Leakage of chyle is associated with increased mortality due to significant loss of essential proteins, immunoglobulins, fat, vitamins, electrolytes, and water (31). For example, the mortality of chylothorax post-esophagectomy can reach 50% (32). These poor outcomes, particularly for patients with a chyle leak post-esophagectomy, served as an impetus for early and aggressive treatment to improve patient outcomes (33).
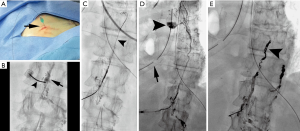
In 1998, Cope demonstrated the safety and feasibility of lymphangiography to facilitate percutaneous trans-abdominal puncture of retroperitoneal lymphatics (34). After passing a microwire into the lymphatic circulation, a microcatheter can be inserted to facilitate lymphatic imaging and subsequently perform thoracic duct embolization to mechanically occlude the site of injury and its inflow (Figure 3). These reports culminated in a 2002 retrospective study of 42 patients with chylothorax in which clinical success after thoracic duct embolization was achieved in over 70% (35). Multiple authors from various institutions have since contributed large patient series since this initial manuscript, in both pediatric and adult populations (36-39). A recent meta-analysis of nine studies on lymphatic interventions for chylothorax from 2008–2017 included 407 patients and found that a pooled clinical success rate of thoracic duct embolization approached 80% with a pooled major complication rate of 2.4% (40). Based on these results, the American College of Radiology recommends lymphangiography and thoracic duct embolization in the treatment planning of chylothorax and as an effective, minimally invasive alternative to surgery (28). Different approaches to access the central lymphatics including direct trans-cervical and retrograde trans-venous approaches have now been described, further increasing the probability of technical success and extending the breadth of possible interventions to include balloon occlusion and stent placement within the thoracic duct (41-44). Given the widespread acceptance of thoracic duct embolization, increased clinical application and innovation is likely.
Chylous ascitesOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
A report of tuberculous chylous ascites in a 2-year-old stands as the initial description of this uncommon condition (15). While chylous ascites was estimated to occur once in every 50,000–187,000 hospital admissions through the 1930–1950s, a single institution review estimated the incidence at nearly 1 per 11,589 in the 1970s (8,45). Patients present with symptoms of ascites including disproportionate weight gain, abdominal discomfort or fullness, dyspnea, or leakage of milky fluid from drains or incisions. Chylous ascites fluid analysis is similar to chylothorax. Underlying etiologies may be traumatic or non-traumatic, which along with daily output, guide therapy. In general, chylous ascites due to malignancy has a poor prognosis compared with non-malignant etiologies, which have a 40% 1-year mortality (8,45).
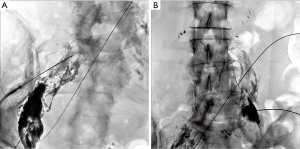
Since the initial work by Cope in 1998 to treat chylothorax and chylous ascites, hundreds of patients have been reported in the literature with successful lymphatic embolization for chylothorax (35-40). In contrast, the published experience for percutaneous image-guided interventional treatment of chylous ascites has been scarce and comprises fewer than 150 patients, many in case reports or small series. There has been an increased interest in image-guided therapies for post-operative chylous ascites, with three recently published retrospective series each with greater than 20 patients (11,12,46). In total, 63 patients with iatrogenic chylous ascites were reported having undergone lymphangiography and a variety of lymphatic embolization techniques and approaches with a collective clinical success rate exceeding 80% with only one major complication, transient hypoglycemia, reported (46). A recent manuscript reviewed the collective literature for image-guided treatment of traumatic chylous ascites and found 18 other manuscripts in addition to their cohort detailing treatment of 96 patients (46). Procedure specific information was available on 82 of the patients, with a leak visualized in 60 of the procedures (73%). Lymphangiography without embolization was performed in 40 of 82 patients with cessation of leakage in 28 of 40 patients (70%). Embolotherapy with coils, glue, or sclerosants was performed in the 42 of 82 patients with clinical success in 37 of 42 patients (88%). Although reporting bias is present in technical notes and case reports, the potential for successful treatment, particularly in patients who underwent diagnostic lymphangiography without therapeutic intent cannot be discounted. Generally, pedal or intranodal lymphangiography is followed through the pelvis and retroperitoneum to evaluate for a lymphatic injury. Often, lymphangiography alone is diagnostic and therapeutic (Figure 4). Injuries below the lumbar spine may be difficult to access with a wire and microcatheter, but needle-based glue embolization may be performed from adjacent inflow lymph nodes if necessary (Figure 5). An advanced technique, balloon occluded retrograde abdominal lymphatic embolization, can be used in recalcitrant cases and involves retrograde lymphatic access from the left venous angle, balloon occlusion near the level of injury, and subsequent sclerotherapy (43). Generally, the experience with treatment of chylous ascites will continue to grow as caregivers realize the potential for image-guided minimally invasive treatment options in this difficult to treat patient population.

LymphoceleOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
In contrast to chylothorax and chylous ascites, which were both described in the 17th century, the first report of lymphocele dates to 1950 (47). Functionally, lymphoceles are a collection of lymphatic fluid encased within a fibrotic wall without a true epithelial lining, histologically differentiating them from a cyst. Lymphoceles are most commonly iatrogenic, occurring after pelvic or retroperitoneal lymphadenectomy during renal transplantation or urologic/gynecologic malignancy staging (48). Surgical exposure of the groin for vascular access, bypass grafting, and extracorporeal membrane oxygenation cannulation are all known causative procedures as well (49). The incidence and location of lymphocele formation varies widely based on the extent of lymphadenectomy, open versus laparoscopic approach, and surgical technique. To achieve diagnosis and differentiate a lymphocele from a seroma or hematoma, the anechoic fluid filled contents are aspirated, which normally yield clear or light, yellow fluid with a lymphocyte predominance on cell count (>70%) and low creatinine level (50). While most lymphoceles are asymptomatic, particularly if small, larger lymphoceles are more likely to have a wide range of symptoms related to structures they compress including ureters, veins, rectosigmoid colon, nerves, or the bladder.
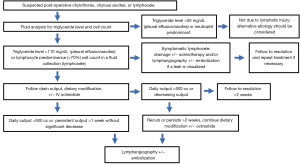
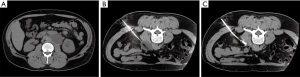
Historically, treatment of lymphoceles was achieved surgically through open and subsequently laparoscopic internal marsupialization or external drainage (48). Since the 1990s, percutaneous aspiration and drainage has been performed and later combined with transcatheter sclerotherapy with success rates equivalent to or higher than surgery without anesthesia allowing treatment on an outpatient basis with faster recovery (49). Using CT or US guidance, a needle is directed into the lymphocele and after confirming location, a wire is placed over which the needle is exchanged for a drain (Figure 6). The fluid is aspirated to collapse the cavity and a variety of sclerosants may be instilled ranging from ethanol and povidone-iodine (the two most common) to bleomycin, doxycycline or many others depending on operator preference. Success rates for ethanol range from 88–97% and for povidone-iodine from 62–89%. Repeat treatment increases overall success rates above 90% and can be performed on an outpatient basis as well (49). Uncommonly, a recalcitrant lymphocele may recur despite drainage and sclerotherapy. Recent reports describing lymphangiography and lymphatic vessel embolization are documenting success (51). In this approach, lymphangiography is performed to reveal an area of leak into the lymphocele and glue embolization is either performed through the inflow lymph node or lymphatic vessel. Several case series have been published revealing success rates in excess of 80% for recalcitrant lymphoceles with few major complications documented (51,52). Treatment algorithms for post-operative lymphatic leaks are shown in Figure 7.
ConclusionsOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
Iatrogenic lymphatic leaks may result in chylothorax, chylous ascites, or lymphoceles and are a consequence of oncologic surgeries. The evolution of lymphangiography and lymphatic interventions allows for successful diagnosis and treatment in the large majority of post-surgical leaks with minimal risk to patients. Consideration should be given to the early interventional treatment to hasten patient recovery and potentially improve outcomes.
AcknowledgmentsOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
None.
FootnoteOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ReferencesOther Section
- Introduction
- Lymphatic anatomy
- Lymphangiography
- Chylothorax
- Chylous ascites
- Lymphocele
- Conclusions
- Acknowledgments
- Footnote
- References
- Cerfolio RJ, Allen MS, Deschamps C, et al. Postoperative chylothorax. J Thorac Cardiovasc Surg 1996;112:1361-5; discussion 1365-6. [Crossref] [PubMed]
- Dougenis D, Walker WS, Cameron EW, et al. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet 1992;174:501-6. [PubMed]
- Weniger M, D’Haese JG, Angele MK, et al. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Amer J Surg 2016;211:206-13. [Crossref] [PubMed]
- Madura JA, Dunbar JD, Cerilli GJ. Perirenal lymphocele as a complication of renal transplantation. Surgery 1970;68:310-3. [PubMed]
- Khauli RB, Stoff JS, Lovewell T, et al. Post-transplant lymphoceles: a critical look into the risk factors, pathophysiology and management. J Urol 1993;150:22-6. [Crossref] [PubMed]
- Donohue RE, Mani JH, Whitesel JA, et al. Intraoperative and early complications of staging pelvic lymph node dissection in prostatic adenocarcinoma. Urology 1990;35:223-7. [Crossref] [PubMed]
- Kay R, Fuchs E, Barry JM. Management of postoperative pelvic lymphoceles. Urology 1980;15:345-7. [Crossref] [PubMed]
- Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med 1982;96:358-64. [Crossref] [PubMed]
- Maldonado F, Cartin-Ceba R, Hawkins FJ, et al. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci 2010;339:314-8. [Crossref] [PubMed]
- Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol 1999;10:1248-54. [Crossref] [PubMed]
- Nadolski GJ, Chauhan NR, Itkin M. Lymphangiography and Lymphatic Embolization for the Treatment of Refractory Chylous Ascites. Cardiovasc Intervent Radiol 2018;41:415-23. [Crossref] [PubMed]
- Hur S, Shin JH, Lee IJ, et al. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol 2016;27:1177-86.e1. [Crossref] [PubMed]
- Dori Y, Keller MS, Rome JJ, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 2016;133:1160-70. [Crossref] [PubMed]
- Itkin M, Piccoli DA, Nadolski G, et al. Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol 2017;69:2929-37. [Crossref] [PubMed]
- Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery 2000;128:761-78. [Crossref] [PubMed]
- Carvalho R, Rodrigues A, Pereira S. La Mise en évidence par la radiographie du système lymphaticque chez le vivant. Ann Anat Path 1931;8:193-7.
- Kinmonth JB, Taylor GW, Harper RK. Lymphangiography: a technique for its clinical use in the lower limb. Br Med J 1955;1:940-2. [Crossref] [PubMed]
- Fischer HW, Zimmerman GR. Roentgenographic visualization of lymph nodes and lymphatic channels. Amer J Roentgenol 1959;81:517-34. [PubMed]
- Kos S, Haueisen H, Lachmund U, et al. Lymphangiography: forgotten tool or rising star in the diagnosis and therapy of postoperative lymphatic vessel leakage. Cardiovasc Intervent Radiol 2007;30:968-73. [Crossref] [PubMed]
- Guermazi A, Brice P, Hennequin C, et al. Lymphography: an old technique retains its usefulness. Radiographics 2003;23:1541-58. [Crossref] [PubMed]
- Kawasaki R, Sugimoto K, Fujii M, et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol 2013;201:659-66. [Crossref] [PubMed]
- Rajebi MR, Chaudry G, Padua HM, et al. Intranodal lymphangiography: feasibility and preliminary experience in children. J Vasc Interv Radiol 2011;22:1300-5. [Crossref] [PubMed]
- Nadolski GJ, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol 2012;23:613-6. [Crossref] [PubMed]
- Krishnamurthy R, Hernandez A, Kavuk S, et al. Imaging the central conducting lymphatics: initial experience with dynamic MR lymphangiography. Radiology 2015;274:871-8. [Crossref] [PubMed]
- Pimpalwar S, Chinnadurai P, Chau A, et al. Dynamic contrast enhanced magnetic resonance lymphangiography: Categorization of imaging findings and correlation with patient management. Eur J Radiol 2018;101:129-35. [Crossref] [PubMed]
- McCarthy HH, Organ CA. Chyloperitoneum. AMA Arch Surg 1958;77:421-32. [Crossref] [PubMed]
- Doerr CH, Allen MS, Nichols FC 3rd, et al. Etiology of chylothorax in 203 patients. Mayo Clin Proc 2005;80:867-70. [Crossref] [PubMed]
- Majdalany BS, Murrey DA Jr, Kapoor BS, et al. ACR Appropriateness Criteria: Chylothorax Treatment Planning. J Am Coll Radiol 2017;14:S118-26. [Crossref] [PubMed]
- Nix JT, Albert M, Dugas JE, et al. Chylothorax and chylous ascites: a study of 302 selected cases. Am J Gastroenterol 1957;28:40-53; discussion 53-5. [PubMed]
- Maldonado F, Hawkins FJ, Ryu JH, et al. Pleural fluid characteristics of chylothorax. Mayo Clin Proc 2009;84:129-33. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007;32:362-9. [Crossref] [PubMed]
- Pillay TG, Singh B. A review of traumatic chylothorax. Injury 2016;47:545-50. [Crossref] [PubMed]
- Merigliano S, Molena D, Ruol A, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 2000;119:453-7. [Crossref] [PubMed]
- Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol 1998;9:727-34. [Crossref] [PubMed]
- Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol 2002;13:1139-48. [Crossref] [PubMed]
- Itkin M, Kucharczuk JC, Kwak A, et al. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 2010;139:584-9. [Crossref] [PubMed]
- Pamarthi V, Stecker MS, Schenker MP, et al. Thoracic duct embolization and disruption for treatment of chylous effusions: experience with 105 patients. J Vasc Interv Radiol 2014;25:1398-404. [Crossref] [PubMed]
- Yannes M, Shin D, McCluskey K, et al. Comparative Analysis of Intranodal Lymphangiography with percutaneous intervention for postsurgical chylous effusions. J Vasc Interv Radiol 2017;28:704-11. [Crossref] [PubMed]
- Majdalany BS, Saad WA, Chick JFB, et al. Pediatric Lymphangiography, Thoracic Duct Embolization, and Thoracic Duct Disruption: a Single Institution Experience in 11 Patients with Chylothorax. Pediatr Radiol 2018;48:235-40. [Crossref] [PubMed]
- Kim PH, Tsauo J, Shin JH. Lymphatic interventions for chylothorax: a systematic review and meta-analysis. J Vasc Interv Radiol 2018;29:194-202.e4. [Crossref] [PubMed]
- Mittleider D, Dykes T, Cicuto K, et al. Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol 2008;19:285-90. [Crossref] [PubMed]
- Guevara CJ, Rialon KL, Ramaswamy RS, et al. US-guided, direct puncture retrograde thoracic duct access, lymphangiography, and embolization: feasibility and efficacy. J Vasc Interv Radiol 2016;27:1890-6. [Crossref] [PubMed]
- Chick JFB, VanBelkum A, Yu V, et al. Balloon-occluded retrograde abdominal lymphangiography and embolization for opacification and treatment of abdominal chylous leakage. J Vasc Interv Radiol 2017;28:616-8. [Crossref] [PubMed]
- Majdalany BS, Khayat M, Sanogo ML, et al. Direct trans-cervical endolymphatic thoracic duct stent-graft for plastic bronchitis. Lymphology 2018;51:97-101. [PubMed]
- Vasko JS, Tapper RI. The surgical significance of chylous ascites. Arch Surg 1967;95:355-68. [Crossref] [PubMed]
- Majdalany BS, Khayat M, Downing T, et al. Lymphatic interventions for isolated, iatrogenic chylous ascites: a multi-institution experience. Eur J Radiol 2018;109:41-7. [Crossref] [PubMed]
- Kobayashi T, Inoue S. Pelvic lymphocyst. Clin Gynecol Obstet 1950;4:91-5.
- Liss MA, Palazzi K, Stroup SP, et al. Outcomes and complications of pelvic lymph node dissection during robotic-assisted radical prostatectomy. World J Urol 2013;31:481-8. [Crossref] [PubMed]
- Karcaaltincaba M, Akhan O. Radiologic imaging and percutaneous treatment of pelvic lymphocele. Eur J Radiol 2005;55:340-54. [Crossref] [PubMed]
- vanSonnenberg E, Wittich GR, Casola G, et al. Lymphoceles: Imaging characteristics and percutaneous management. Radiology 1986;161:593-6. [Crossref] [PubMed]
- Baek Y, Won JE, Chang SJ, et al. Lymphatic embolization for the treatment of pelvic lymphoceles: Preliminary experience in five patients. J Vasc Interv Radiol 2016;27:1170-76. [Crossref] [PubMed]
- Smolock AR, Nadolski G, Itkin M. Intranodal glue embolization for the management of postsurgical groin lymphocele and lymphorrhea. J Vasc Interv Radiol 2018;29:1462-65. [Crossref] [PubMed]