
The value of cystatin C and urinary and serum neutrophil gelatinase-associated lipocalin during the perioperative period of renal transplantation
Introduction
Renal transplantation is the best method for the treatment of end stage of renal disease (ESRD). Patients do not need dialysis after a successful kidney transplant. Benefiting from the efficient system of donors after cardiac death (DCD), the number of renal transplants is rising rapidly in China. The surgical technique for renal transplantation has constantly improved and is now a mature technology. However, the management of the perioperative period is very complicated. Delayed graft function (DGF) is one of the most common complications after renal transplantation, and it is the main problem of successful transplantation. DGF can increase the incidence of acute rejection and decrease the survival time of the allograft (1). In our clinical practice, DGF is diagnosed depending on urine volume, Cr, and the need for dialysis. Early diagnosis of DGF and timely intervention are very important. However, there is currently no valuable indicator to evaluate renal status during the perioperative period.
Neutrophil gelatinase-associated lipocalin (NGAL) is a small protein found in neutrophils, and recent research has confirmed its function in evaluating renal performance. It is markedly increased after ischemic, septic, or nephrotoxic injury of the kidney (2). Other research found that urine NGAL levels were higher in DGF patients. Serum and urinary NGAL may thus be useful early predictors of DGF after kidney transplantation (3,4). Moreover, NGAL is produced in the renal tubule cells and will rise rapidly immediately after acute kidney injury. This can easily be detected in urine samples (5).
Cystatin-C (Cys-C) is an endogenous cysteine proteinase inhibitor, independent of gender, age, and muscle mass of the individual. Cys-C in the blood is cleared only by glomerular filtration, and this feature gives its exclusive sensitivity to glomerular filtration rate (GFR) changes (6). Some researchers have shown that Cys-C is superior to serum creatinine in the assessment of renal function (7). Other recent investigation has indicated that cystatin C might become a sensitive marker in the early diagnose of renal injury (8).
Our study aimed to study the function of NGAL and Cys-C to assess DGF and predict the short-term prognosis of renal transplantation patients.
Methods
This study was performed at the First Affiliated Hospital of Soochow University, Suzhou, China. Our research was a retrospective analysis approved by the medical ethics committee of the First Affiliated Hospital of Soochow University (Number 38). We collected the information of the patients from June 2016 to July 2017. All the patients were first time recipients of renal transplantation, needed because of ESRD. This study comprised 47 patients. The mean age of the patients was 44 years old.
Laboratory methods
Urine and serum samples were collected on the morning following the operation, nearly 3 to 12 hours after renal transplantation, then daily during the 1st week following the operation, and then at 14 and 21 days after operation. Serum creatinine was examined preoperatively, daily until the 2nd week, and then weekly until discharge.
NGAL was measured with an NGAL kit (Getein Biotech, China) with a detection range of 50–5,000 ng/mL. If the value of the sample was higher than 5,000 ng/mL, we diluted the sample 2 times. If the value was lower than 50 ng/mL, we marked it 50 ng/mL. NGAL measurements were performed using a dry immunofluorescence method. Cys-C was measured with cystatin C kit (Meikang Biotech, China); at the same time points, NGAL was measured. The test method was latex-enhanced immunoturbidimetry. The laboratory technician was blinded to patient information. Creatine was tested with enzymatic creatine-2 reagents (Siemens Healthcare Diagnostics Inc., Canada); the test is based on an enzyme method.
Definitions
DGF was defined as the requirement for dialysis within the first 7 days after renal transplantation, due to the poor recovery of the graft. Situations such as hyperkalemia and hypervolemia were excluded (9). Non-DGF was defined as no dialysis requirement within the first week after renal transplantation (10).
S-NGAL means the NGAL in serum and u-NGAL means the NGAL in the urine. S-Cr means the creatinine in serum.
Estimated GFR was calculated using the correction formula known as the Chinese population corrected MDRD, which has better accuracy of the true GFR in Chinese renal transplantation patients (11).
The standard of discharge was when renal function and the concentration of antirejection drugs were stable. There were no infections such as urinary tract infection or pneumonia. The wound healed well, and the internal stent was extracted.
Statistical analysis
Statistical analysis was done using SPSS 19.0. The diagrams of the changes in the variables were drawn with Graphpad 7.0. Normality was evaluated for each variable by the Kolmogorov-Smirnov test. The data are summarized as the mean and standard deviation (SD) for variables with a normal distribution or as the median and 25th–75th quartiles (interquartile range) for variables with a skewed distribution. Comparisons between continuous variables were made using either the t-test or the Mann-Whitney test, according to the data. Categorical variables are presented as percentages. The Chi-square test was used to compare the differences in the categorical variables. Receiver-operating characteristic (ROC) curves and area under the curve (AUC) were used for determining the efficiency and cut-off point of s-NGAL, u-NGAL, Cys-C, and s-Cr, in diagnosing DGF. All the analyses were two-tailed with a significance level of 0.01. The best cut-off values for biomarkers and s-Cr were chosen according to the maximum sum of sensitivity and specificity. Multivariable analysis was used to describe the independent association of s-NGAL, u-NGAL, Cys-C, and s-Cr with e-GFR at discharge.
Results
Patient characteristics
Basic clinical characteristics of the renal transplantation patients are shown in Table 1. Before the research period, 1 patient suffered from bleeding, and the allograft was resected. Forty-seven patients were enrolled in the study. Among these patients, 11 patients suffered DGF. At basic levels, the 2 groups were compared in terms of donor condition and recipient condition. We used the Kolmogorov-Smirnov test to confirm the normality of the data. Apart from BMI and urine volume before renal transplantation, the other data were normally distributed. There were no differences between the 2 groups except for cold ischemia time (P<0.01) and urine volume before renal transplantation (P<0.01).
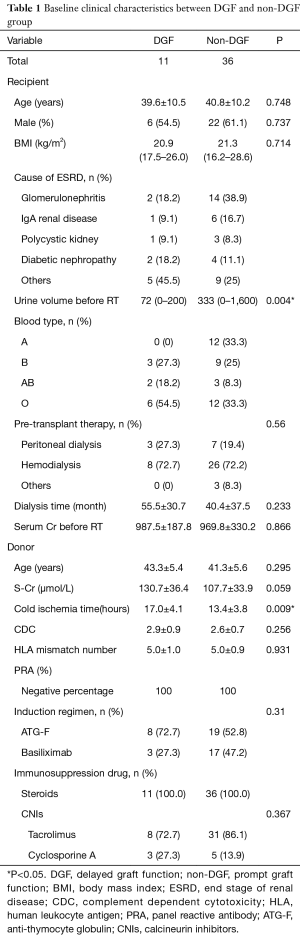
Full table
Differences in s-NGAL, u-NGAL, Cys-C, s-Cr, and eGFR between the DGF and non-DGF groups
The differences in the biomarkers between the two groups are listed in Table 2 and Table 3. We checked the normality of the data first. All the s-NGAL and Cys-C data were normally distributed. However, the u-NGAL, s-Cr, and eGFR data were not normally distributed. We then used the t-test to compare the difference in s-NGAL and Cys-C; the data are shown as the mean ± SD. We used the Mann-Whitney test to compare the difference in u-NGAL, s-Cr, and eGFR; the data are shown as the median (interquartile range). S-NGAL concentrations were markedly higher in the DGF group, except for the time point on the 14th day (P>0.05). The u-NGAL, Cys-C, and s-Cr levels were markedly higher in the DGF group at all time points (P<0.05). The e-GFR level was markedly higher in the non-DGF group at the time of discharge (P<0.01).
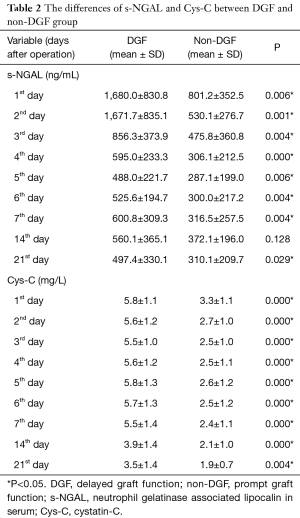
Full table
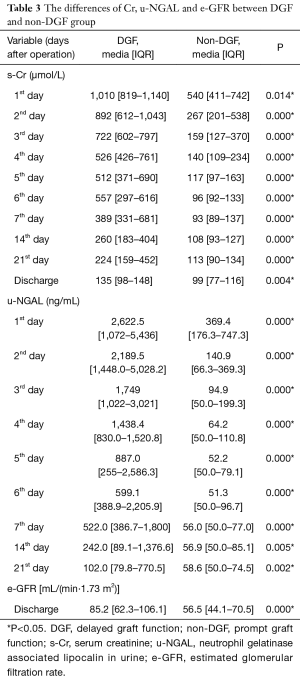
Full table
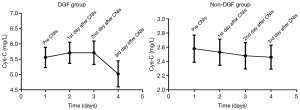
The longitudinal changes in u-NGAL, s-NGAL, Cys-C, and s-Cr were drawn with Graphpad 7.0 and are shown in Figure 1. The longitudinal changes in Cr (Figure 1B), s-NGAL (Figure 1C), and u-NGAL (Figure 1D) were characterized by an initial rapid decline and then a slow decrease in the following days in both the DGF and non-DGF groups. The curves for the DGF group were above the curves of the non-DGF group at all points. The change in Cys-C was the same in the non-DGF group. However, the change in Cys-C in the DGF group was different.
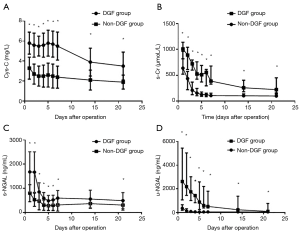
The decrease in Cys-C was slower in the DGF group than the non-DGF group (Figure 1A). We doubt that this was associated with the addition of calcineurin inhibitors (CNIs). We then investigated the longitudinal changes in Cys-C before and after the addition of CNIs. The results are provided in Figure 2. There was a small rise after the addition of CNIs and then a rapid decline on the 3rd day in the DGF group. However, there was no rise in the non-DGF group.
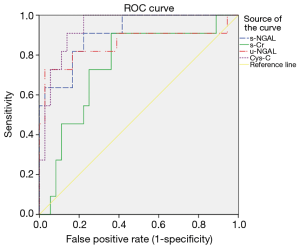
ROC analysis of s-NGAL, u-NGAL, Cys-C, and s-Cr for predicting DGF
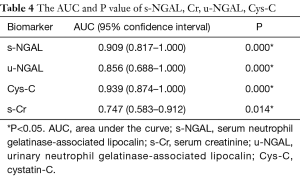
The predictive value of s-NGAL, u-NGAL, Cys-C, and s-Cr was evaluated with ROC curves. The results are shown in Figure 3, Table 4, and Table 5. Table 4 shows the AUC and P value for the four indicators on the 1st after the operation. S-NGAL, u-NGAL, and Cys-C were accurate in predicting DGF, while s-Cr was not (P>0.01); Cys-C shows the largest AUC (0.939). The cut-off values for s-NGAL, u-NGAL, and Cys-C were chosen according to the maximum sum of sensitivity and specificity (Table 5). The data in the table contain the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). S-NGAL had the highest sensitivity at 1,025.86 ng/mL (sensitivity: 0.909; specificity: 0.778; PPV: 0.556; NPV: 0.966). U-NGAL had the highest specificity at 1,787.8 ng/mL (sensitivity: 0.727; specificity: 0.972; PPV: 0.889; NPV: 0.921). Cys-C had the highest sum of sensitivity and specificity at 4.77 mg/L (sensitivity: 0.818; specificity: 0.889; PPV: 0.692; NPV: 0.941).
Full table

Full table
The predictive value of s-NGAL, u-NGAL, Cys-C, and s-Cr for predicting renal function at discharge
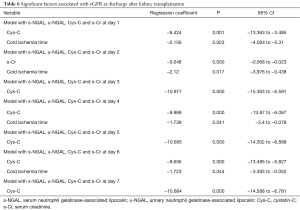
The predictive values of s-NGAL, u-NGAL, Cys-C, and s-Cr were tested with multivariable analysis. In multivariable linear regression models, we incorporated 12 variables including recipient sex, age, BMI, s-Cr before the operation, CDC, cold ischemia time, acute rejection, drug poisoning, s-NGAL, u-NGAL, Cys-C, and s-Cr after the operation. The s-NGAL, u-NGAL, Cys-C, and s-Cr values during the first 7 days after operation were analyzed. We used the eGFR at the time of discharge as the prognostic value. The results are shown in Table 6. Our research shows that the values of Cys-C on days 1, 3, 4, 5, 6, and 7 after operation were independently associated with eGFR at discharge (P<0.01). Cys-C and eGFR are negatively related. The value of s-Cr on day 2 was independently associated (negative relationship) with eGFR at discharge (P<0.01). Cold ischemia time of the organ was independently associated (negative relationship) with eGFR at discharge (P<0.05).
Full table
Discussion
DGF is a common complication after renal transplantation. The diagnostic criteria of DGF depend on the need for dialysis, diuresis, and serum creatinine level (12). Some research has tried to find predictive models to help diagnose the condition, but they were not so efficient due to the complex influences and poor accuracy (1). New markers have been developed in recent years to evaluate renal status after renal transplantation. NGAL and Cys-C are both new measures of the study. More and more research has been done to study their function during the perioperative period of renal transplantation. However, little research has compared the differences between them. Our study found the following: (I) s-NGAL, u-NGAL and Cys-C could reflect renal function sensitivity and that the patients in the DGF group were more sensitive to CNIs as reflected by Cys-C; (II) Cys-C had the highest sum of sensitivity and specificity at 4.77 mg/L, with a sensitivity of 0.818 and a specificity of 0.889; (III) Cys-C can predict the short-term prognosis of renal transplantation patients.
Cys-C is a polypeptide chain with 120 amino acids and can only be filtered by the glomerulus. It is reabsorbed by the proximal tubule (13). In the past, Cys-C was used to assess the GFR. Recently, a few researchers have begun to study the function as a predictor of renal injury. Some research shows that Cys-C is not superior to creatinine for the detection of acute renal dysfunction (14), while other research shows that Cys-C can predict renal injury (15). Our findings indicate that Cys-C can not only predict DGF but can also predict renal function at discharge, as the efficiency was superior to creatinine and NGAL. Other investigations have compared the value of creatinine on the 2nd day or the 3rd day after operation for predicting DGF; however, we think the most useful value is obtained the 1st day after the operation, because the patient may receive dialysis on subsequent days. When we studied the longitudinal change of Cys-C, we found that the decrease in Cys-C was not very obvious. Then, we compared the changes in Cys-C before and after the use of CNIs. We found that there was a small rise in Cys-C in the DGF group after the use of CNIs and then a rapid decline. This may mean that DGF patients are more sensitive to the nephrotoxicity of CNIs and can recover quickly. This phenomenon supports the point of a kidney transplant guideline recommending that CNIs not be delayed until the graft has recovered (16).
NGAL was isolated for study in the 1990s (17). Until recently, the functions of NGAL were not well understood. Recent research shows that NGAL levels rise immediately after kidney injury and thus can be used as a marker of renal dysfunction. An increasing number of studies have begun to examine its function in renal injury, especially after renal transplantation, and have yielded promising results. Almost all the findings show it is a better indicator than creatinine (3,9,15,18), and our research is in line with this. We discovered that both the NGAL in serum and urine were higher in the DGF group at all the time points; it declined over time in both groups but was continuously higher in the DGF group. Although NGAL is not as good as Cys-C in predicting DGF, urine NGAL is noninvasive, and this constitutes the main advantage of u-NAGL.
Additionally, at the point of the maximum sum of sensitivity and specificity, s-NGAL had the highest sensitivity, and u-NGAL had the highest specificity. Some researchers have studied the predictive function of NGAL. Jafari et al. (18) found that plasma NGAL levels at 2, 24, and 96 hours after transplantation could predict graft loss at 3 months. Fonseca et al. (10) concluded that u-NGAL levels were associated with prognosis at 1 year. Our research studied the predictive function of NGAL and found it was inferior to Cys-C.
In this study, we did not examine the samples before renal transplantation because of the urgency to operate and because a few renal failure patients lacked urine. We chose the sample collected on the first morning after the transplantation as the first value because we thought it was the most practical to collect.
Some studies have shown that these markers can predict the prognosis of three months and one year after kidney transplantation (10,18). However, Pezeshgi et al. (3) showed that serum and urinary NGAL had no relationship with graft loss rate and patient death rate after kidney transplantation. Many factors can influence renal function, and long-term prognosis is complicated to evaluate. Our research explored the function of Cys-C and NGAL in predicting the short-term prognosis which is simple and practical. We found that the value of Cys-C on days 1, 3, 4, 5, 6 and 7 after operation were independently associated with eGFR, the same as cold ischemia time. To our knowledge, this has never been reported before.
Similar to the other study (10), we found that cold ischemia time was associated independently with renal function at discharge. We also found that cold ischemia time in the DGF group was longer than that in the non-DGF group. Cold ischemia time is one of the most important causes of DGF, and it can aggravate renal ischemia-reperfusion injury (19). Going forward, we will try to shorten the cold ischemia time to improve the prognosis of renal transplantation. Furthermore, we will introduce the use of a hypothermic machine perfusion system to reduce ischemia-reperfusion injury (20).
Our research still had some limitations. We did not obtain s-NGAL, u-NGAL, and Cys-C levels before renal transplantation. As a next step, we will obtain these values when the patients come for matching. Furthermore, there were only 47 participants in the study, which is a small number. We will expand the sample size and continue to confirm our findings in our following work. As the number of cases increases and follow-up time prolongs, we will study the relationship between these markers and long-term prognosis in the future.
Conclusions
Our research found that Cys-C, s-NGAL, and u-NGAL could accurately reflect the renal function after renal transplantation. Cys-C had the highest sum of sensitivity and specificity at the point of 4.77 mg/L with a sensitivity of 0.818 and a specificity of 0.889 compared with s-NGAL and u-NGAL. The value of Cys-C of the first week after the operation was independently associated with eGFR at discharge and can predict the short-term prognosis of renal transplantation patients.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 81500572).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the medical ethical committees of the First Affiliated Hospital of Soochow University.
References
- Zhang H, Zheng L, Qin S, et al. Evaluation of predictive models for delayed graft function of deceased kidney transplantation. Oncotarget 2017;9:1735-44. [PubMed]
- de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J 2012;5:102-8. [Crossref] [PubMed]
- Pezeshgi A, Abedi Azar S, Ghasemi H, et al. Role of plasma neutrophil gelatinase-associated lipocalin as an emerging biomarker of acute renal failure following kidney transplantation and its correlation with plasma creatinine. J Renal Inj Prev 2016;5:98. [Crossref] [PubMed]
- Maier HT, Ashraf MI, Denecke C, et al. Prediction of delayed graft function and long-term graft survival by serum and urinary neutrophil gelatinase–associated lipocalin during the early postoperative phase after kidney transplantation. PLoS One 2018;13:e0189932. [Crossref] [PubMed]
- Mishra J, Mori K, Ma Q, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2004;15:3073-82. [Crossref] [PubMed]
- Pöge U, Stoschus B, Stoffel-Wagner B, et al. Cystatin C as an endogenous marker of glomerular filtration rate in renal transplant patients. Kidney Blood Press Res 2003;26:55-60. [Crossref] [PubMed]
- Roos JF, Doust J, Tett SE, et al. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children-A meta-analysis. Clin Biochem 2007;40:383-91. [Crossref] [PubMed]
- Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med 2009;37:553-60. [Crossref] [PubMed]
- Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant 2008;23:2995-3003. [Crossref] [PubMed]
- Fonseca I, Oliveira JC, Almeida M, et al. Neutrophil gelatinase-associated lipocalin in kidney transplantation is an early marker of graft dysfunction and is associated with one-year renal function. J Transplant 2013;2013:650123.
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-44. [Crossref] [PubMed]
- Tomlanovich S, Golbetz H, Perlroth M, et al. Limitations of Creatinine in Quantifying the Severity of Cyclosporine-Induced Chronic Nephropathy. Am J Kidney Dis 1986;8:332-7. [Crossref] [PubMed]
- Malyszko J, Lukaszyk E, Glowinska I, et al. Biomarkers of delayed graft function as a form of acute kidney injury in kidney transplantation. Sci Rep 2015;5:11684. [Crossref] [PubMed]
- Slort PR, Ozden N, Pape L, et al. Comparing cystatin C and creatinine in the diagnosis of pediatric acute renal allograft dysfunction. Pediatr Nephrol 2012;27:843-9. [Crossref] [PubMed]
- Hošková L, Franekova J, Málek I, et al. Comparison of Cystatin C and NGAL in Early Diagnosis of Acute Kidney Injury After Heart Transplantation. Ann Transplant 2016;21:329-45. [Crossref] [PubMed]
- Baker RJ, Mark PB, Patel RK, et al. Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol 2017;18:174. [Crossref] [PubMed]
- Kjeldsen L, Johnsen AH, Sengeløv H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425-32. [PubMed]
- Jafari A, Khatami MR, Dashti-Khavidaki S, et al. Plasma Neutrophil Gelatinase-Associated Lipocalin as a Marker for Prediction of 3-Month Graft Survival after Kidney Transplantation. Int J Organ Transplant Med 2017;8:17-27. [PubMed]
- Saat TC, van den Akker EK, IJzermans JN, et al. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med 2016;14:20. [Crossref] [PubMed]
- Kox J, Moers C, Monbaliu D, et al. The benefits of hypothermic machine preservation and hot cold ischemia times in deceased donor kidneys. Transplantation 2018;102:1344-50. [Crossref] [PubMed]


