
Contemporary techniques and outcomes of robotic assisted radical cystectomy with intracorporeal urinary diversion
Introduction
Radical cystectomy remains one of the highest morbidity oncologic surgeries (1-3). The gold standard for muscle invasive bladder cancer (MIBC) and high-risk non-muscle invasive bladder cancer (NMIBC) refractory to intravesical treatments is open radical cystectomy (ORC) with extended pelvic lymph node dissection (PLND) (4). In recent years, robotic assisted radical cystectomy (RARC) has increased in popularity, mirroring the trend that was seen for radical prostatectomy and partial nephrectomy (5,6). Surgeons adopting the robotic technique enjoy enhanced ergonomic advantages as well as three-dimensional vision during surgery (7); however, debate remains as to whether RARC preserves benefits of ORC in terms of adequate cancer control following bladder extirpation (8). Unlike that seen with other morbid surgical procedures, for example, the Whipple procedure, oncologic equivalence of RARC and ORC has been shown using randomized clinical trials (9-12). Still, consistent level 1 evidence is needed before it is time to establish superiority of RARC over ORC.
In order to reduce the considerable morbidity associated with the learning curve of a new technique, urinary diversion is typically done extracorporeally with RARC; however, adoption of the totally intracorporeal approach for urinary diversion has seen an 11% increase in use per year since 2005 and is not reserved for high volume centers only (13). Both large and small volume centers have published their studies on RARC with comparative extracorporeal urinary diversion (ECUD) vs. intracorporeal urinary diversion (ICUD), or one of the two modalities independently (14-24).
The results of these studies demonstrate perioperative outcomes of RARC with ICUD. Benefits include shorter length of stay (LOS), reduced physiological stress and analgesic requirement as well as decreased blood loss and quicker return of bowel function. In fact, one recent analysis showed RARC to have a shorter operative time (OT) than ORC (13). RARC with ICUD is not without disadvantages and debate remains as to whether or not ICUD offers the same benefits as ECUD (25).
Background
Beecken et al. described the first robotic-assisted laparoscopic radical cystectomy with ICUD (26). The authors reported an 8.5-hour OT with 200 mL blood loss. Following a 10-year gap in which surgeons modified techniques and gained experience with the robotic approach, Roswell Park reported a large cohort of ICUD in 2012 (27) followed by two larger cohorts from the Karolinska Institute in 2013 (28,29). Azzouni et al. published the largest cohort of intracorporeal ileal conduit (ICIC) in 2013 (21) followed by Desai et al. who published the largest cohort of intracorporeal neobladder (ICNB) in 2014 (30). During this period, there were numerous reports on RARC with ECUD (31). Large studies comparing ICUD and ECUD report comparative perioperative outcomes while acknowledging increased complexity of ICUD (13,18).
Fifteen years after its introduction, RARC is still in its relative infancy but has become popular amongst robotic surgeons. Thus, focus has shifted toward accumulating data comparing ICUD and ECUD (7). Herein, we review those studies comparing ICUD and ECUD for ileal conduit (IC), neobladder (NB) and continent cutaneous diversion (CCD).
Techniques
Radical cystectomy with ICIC
As surgeons worldwide gain more experience with RARC, the technique for performing intracorporeal diversion has evolved. Here we will focus on techniques and technical pearls (Table 1) for performing this challenging procedure.

Full table
Patient selection
As with any complex surgical procedure, patient selection is a key process in helping to ensure a successful operation and favorable outcome. Patients without prior abdominal surgery or radiation, with favorable body mass index (BMI) (<30 kg/m2), non-bulky disease, minimal to no cardiovascular or pulmonary disease, and good performance status tend to facilitate a favorable operation (32). Certainly, as one begins the transition, selecting patients with as many of these factors as possible is good practice. In converting from ECUD, surgeons with significant robotic experience and comfort in pelvic surgery can transition smoothly with favorable OTs and outcomes, even with these factors present. For those who have already performed many robotic radical cystectomies with ECUD, it is likely many patients had higher BMI, prior radiation and bulky disease. Thus, experiences with these challenges are not lacking (34).
Positioning and port placement
Positioning of the ports should be no different than typically performed with robotic radical prostatectomy. Many safe and effective methods have been well described in the literature, with the majority using a 30° Trendelenburg approach.
Karolinska Institute and City of Hope Cancer Center describe the two major port configurations (Table 2). The first centers around a port placed 5 cm above the umbilicus, and remaining robotic ports placed at the level of the umbilicus. The 4th arm is double cannulated through a left-sided 15 mm port just above the left anterior superior iliac spine (ASIS), with the second assistant port placed between the right-sided working robotic arm and the optic port. The City of Hope approach places ports based off of measurements from the symphysis pubis (Figure 1). Our approach is for port placements to mirror robotic radical prostatectomy with the modification of placing ports 2–5 cm higher in the abdomen to facilitate extended PLND. The addition of a 15 mm assistant port, to facilitate passage of a stapler for vascular control of the bladder pedicles (a significant time-saver over use of energy-based vessel control) and large Endocatch (US Surgical) bag, is easily closed at the end of the case with an interrupted stitch or Carter-Thomason device. Azzouni and colleagues use the addition of a suprapubic 12 mm port after the bladder has been placed in the Endocatch (US Surgical) bag to facilitate favorable direct access for side-to-side bowel anastomosis without creating torsion on the bowel towards lateral assistant ports. This becomes a convenient spot for bag removal yielding a minimal Pfannenstiel incision (21).
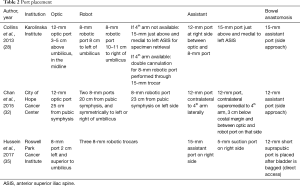
Full table
ICIC creation
Following cystectomy, the bowel segment is measured as in open surgery. Two techniques for bowel manipulation are described. The Marionette technique uses a silk Keith needle to suspend the bowel in the right lower quadrant and through the full thickness of the bowel in the future distal end of the conduit. This can be raised and lowered to facilitate isolation of the conduit and later positioning for the ureteroenteric anastomosis (16,21). The second uses Ligaloop bands positioned in between windows in the bowel mesentery. We have found a time and potential cost-saving tip dividing the bowel and mesentery with a staple load to avoid needing a robotic vessel sealer or other energy device. The side-to-side anastomosis is performed via the side or aforementioned suprapubic port with a 45 or 60 mm stapler (we prefer the latter). The transverse staple line closure is completed using the side port as it facilitates a direct approach.
The conduit is created using either a Bricker or Wallace approach to the uretreoenteric anastomosis. The literature appears to show equivalent stricture rates between the two techniques (Table 3). Thus, surgeon preference can dictate selection with the knowledge that Wallace anastomoses may facilitate contralateral seeding in instances of upper tract recurrence or the need to reimplant a healthy ureter if only one side is strictured. Single J diversion stent placement is facilitated by the right-sided assistant port entering the distal aspect of the conduit through a hollow sucker or using Cardiere forceps to pull through the ileal limb, and subsequently into each renal unit. The anastomosis is typically completed using a running suture but certainly can be interrupted with absorbable 4-0 standard or barbed suture. The stoma is created in a preplanned location. We typically use the right-sided working robotic arm site and use a bowel grasper to hold the distal end of the conduit and stents to facilitate passage through the abdominal wall. Others have placed a 12 mm port at the stoma site to do the same (35).

Full table
Intracorporeal continent diversion
The majority of published data describes intracorporeal NB over CCD (32,40). Both Studer (41) and Hautmann (35) techniques are described in great detail (Table 4). Techniques focus on creation of the urethral-enteric anastomosis prior to any bowel division. The purpose of performing this step first is to aid in bowel retraction for sewing of the ileal pouch. With this in mind, Guru and colleagues have recently adapted the Marionette stich from their previous technique for IC creation to NB creation by suturing the two limbs of ileum to a Foley catheter during W-pouch formation for dynamic retraction (personal observation). Following urethral creation/fixation, the bowel is detubularized along the antimesenteric border for creation of the pouch. For both Studer and W-pouch creation, the posterior plate is formed first, followed by restoration of bowel continuity, excision of the staple lines, and ureteroenteric anastomosis with anterior closure as the final step. Both barbed and unbarbed absorbable sutures have been described (16,24,41).
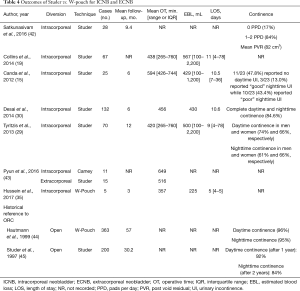
Full table
Outcomes
Oncologic adequacy of RARC
Over the last several years, randomized clinical trials and retrospective studies have consistently shown equivalence of RARC to ORC in terms of perioperative and functional variables as well as complication rates and recovery times. There has been no overt evidence, however, of oncological superiority of RARC over ORC (9,23,46-48). In the first randomized clinical trial to assess long-term oncological outcomes between the two modalities, the large, multi-institutional, RAZOR trial showed noninferiority of RARC over ORC. Two-year progression-free survival was 72.3% in RARC vs. 71.6% in ORC. Rates of local recurrence were low in both RARC and ORC groups (4% vs. 3%, respectively) and the study showed no statistically significant differences between groups in lymph node yield, positive surgical margins, complication rates, estimated blood losses (EBLs) or quality of life (12).
Safety and feasibility of RARC remain a concern for surgeons considering this technique. Nguyen et al. reported on high rates of peritoneal carcinomatosis in their retrospective cohort of RARC (n=263) vs. ORC (n=120) patients from 2001–2014. Rates of peritoneal carcinomatosis were 9/43 (21%) vs. 2/26 (8%) in those with distant recurrence for RARC and ORC, respectively. A possible hypothesis suggested from this paper for unexpected recurrence patterns was lymphatic spreading during robotic surgery due to the robotic technique; however, it was suggested that peritoneal metastasis was more a result of cancer biology than of surgical technique (49). Conversely, Collins et al. reported a rate of 0.7% peritoneal carcinomatosis and 0.3% port site metastasis in a cohort of 717 patients who received RARC between 2003–2015 (48). Similarly, an analysis of the IRCC database of patients receiving RARC during the same time period showed a 1.0% rate of peritoneal carcinomatosis and a 0.4% rate of port site metastases. Promising results from this group were low incidence of early oncologic failure with RARC as well as decreasing rates of early oncologic failure over time (50). Other investigations of RARC populations found no incidence of peritoneal carcinomatosis (51,52).
The use of RARC with ICUD does not appear to compromise PLND. In 2013, Marshall et al. performed an analysis of 765 RARC patients from the IRCC database for receipt of PLND. In all, 58%, 40% and 2% of patients underwent extended, standard and no PLND, respectively. Mean lymph node yield was 21, 13 and 18 nodes in extended, standard and overall PLND, respectively. Stratified by location of diversion, 66% and 65% of patients who had ICUD and ECUD, respectively, underwent an extended PLND. Stratified by type of diversion, 61% of patients who received IC underwent extended PLND while 52% of patients who received NB underwent an extended PLND. Institutional volume and sequential case number were predictors of extended PLND with a reported four-fold likelihood of extended PLND by surgeons’ 51st case (53).
Perioperative outcomes
The following sections include perioperative outcomes of ICUD and ECUD stratified by IC, NB and CCD. Outcomes include OT, diversion time (DT), EBL, conversion rate, LOS and time to flatus.
IC
Nearly all ICIC OTs in the current literature remained within the recommendations by the Pasadena Consensus Panel of <7 hours (7). OTs reported by large cohort studies did not appear to differ between the intracorporeal and extracorporeal approach, indicating increased experience worldwide (Table 5) (20,22,39,52). DTs and EBL for ICIC and extracorporeal ileal conduit (ECIC) series ranged from 92–200 (21,22,33) and 120 (22) minutes and 200–400 (3,20-22,33,54) and 300–525 (20,22,39,52) mL, respectively. Where reported, extreme blood losses were attributed to extended PLND for ICIC diversion (54) and external iliac vein injury as well as severe adhesion for ECIC diversion (39). Conversion rates in ICIC series were between 4–5% (20). Conversions were due to locally advanced, high-grade disease as well as intraperitoneal adhesions resulting in bowel injury and subsequent conversion (3). LOS was similar between ICIC and ECIC groups (3,21,22,33,54). Pasadena Consensus Panel suggests LOS of 5–10 days for surgeons of all experience levels (7). Time to flatus was seldom reported; however, in a single surgeon study by Kang et al., return to bowel function was 2.5 days for both ICIC and ECIC (39). In a multi-institutional analysis, median [range] was 3.5 [2–8] days for ECIC (22). Time to flatus in randomized clinical trials was 3–4 and 2–4 days for ORC and RARC, respectively (23,55).

Full table
Orthotopic NB
While OTs were longer for the extracorporeal approach, the largest cohort series of ICNB (n=132) and extracorporeal neobladder (ECNB) (n=91) had comparable OTs (Table 6). Tyritzis and colleagues report a direct transition from ORC to RARC with ICUD in their NB cohort of 70 patients. This is evident in their OT as well as in other perioperative metrics (29). DTs for ICNB and ECNB were both between 124–300 minutes (22,33,56,57). EBLs were similar when comparing the largest series of ICNB (29,30,57) and ECNB (52,59), with both diversion types ranging from about 380–550 mL. Conversion rates reported for ICNB were between 4.0–5.7%. Reasons for conversion to open diversion included technical difficulties, long OTs related to construction of a reservoir and anastomotic insufficiency between the urethra and the reservoir (29,54). LOS ranged from 5–14 and 9–17 days for ICNB and ECNB, respectively. Improvements in LOS were consistent with increased experience (29,54). Time to flatus varied between 2.5–5.0 (22,56,58) and 2.3–3.2 (22,39) days for ICNB and ECNB, respectively. Time to bowel movement was 6 days, where reported (56).

Full table
Continent cutaneous
In 2017, Desai et al. reported on the largest intracorporeal CCD cohort to date. In their cohort of 10 subjects, mean OT was 6 (range, 5–9) hours, mean DT was 210 (range, 160–315) minutes and there were no conversions to open surgery as well as no bowel leaks or obstructions (40). When comparing this cohort to extracorporeal continent cutaneous cohorts, OT, EBL as well as rates of blood transfusion were notably higher for extracorporeal diversion. LOS was not notably different between the intracorporeal and extracorporeal approach (52,59-61).
So as not to understate the complexity of this procedure, it is important to examine complication and readmission rates following RARC with ICUD and ECUD in light of rates for these metrics in ORC populations. Reported here are rates of blood transfusion, deep venous thrombosis (DVT), stricture and ileus, as well as outcomes regarding neoadjuvant chemotherapy (NAC) and adjuvant chemotherapy (AC), BMI, function and continence, learning curves, enhanced recovery protocols (ERP) and cost-effectiveness in the setting of RARC with ICUD.
Thirty- and 90-d readmission rates
A matched comparison of RARC and ORC showed no difference in 30-d readmissions between groups (62). This finding is not supported by more recent studies comparing ORC and RARC (25). Historically readmission rates for RARC (13,63) and ORC (2,64) have been similar for 30-d, 90-d and overall readmissions.
Thirty and 90-d readmission rates were higher for ICNB than ICIC groups with 30-d readmission rates for ICNB and ICIC reported as 30% and 7–16%, respectively (16,21,56). Ninety-day readmission rates for ICNB and ICIC were 15% and 4%, respectively (21,56). Readmission rates for ECIC and extracorporeal CCD were 23% (16) and 39% (60), respectively, with studies showing ECUD to be associated with higher readmission rates compared to ICUD overall (13,18). Although the mechanism for readmission rates remains unclear, increased readmission could be due to bowel manipulation or prolonged periods of pneumoperitoneum.
Overall complications
Sathianathen et al. recently performed a meta-analysis of randomized controlled trials (including the RAZOR trial) comparing ORC and RARC, and found no difference in 90-d major complications between groups (46). A recent retrospective, multi-institutional analysis of ORC vs. RARC supports this finding (25). Concerns for complication rates by location of diversion are addressed by a recent, retrospective, IRCC analysis of 1,094 and 1,031 patients who underwent ICUD and ECUD, respectively. ICUD was found to have significantly greater rates of overall and high-grade complications one month after operation, with high-grade complications decreasing over time in the ICUD cohort and remaining stable in the ECUD cohort. ICUD and ECUD were with comparable rates of T3 disease, positive nodal disease, lymph node yield, and positive surgical margin status in this study (13). An earlier analysis by Ahmed et al. also showed ICUD to be favorable in terms of gastrointestinal complications and risk of postoperative 90-d complications (18). Comparatively, major and minor complication rates in a large, contemporary cohort of 753 patients undergoing ORC were 7% and 25%, respectively (64).
Desai et al., in the largest reported ICNB cohort of 132 patients, reported highest-grade complications as infective (28.8%), gastrointestinal (7.6%), genitourinary (21.2%), and bleeding and hematologic (7.6%). Fifteen percent of patients experienced high-grade complications at both 30- and 90-d. There were 13 cases of sepsis at 30-d. At 90-d, there were six cases of sepsis, five cases of ureteroenteric stricture, three cases of hydronephrosis, three cases of reservoir stones, and one case each of bladder neck contracture, NB-vaginal fistula, NB-bowel fistula and bowel leak (30).
In 2017, Tan et al. conducted an in-depth critical analysis of surgical and medical complications in a cohort of ICIC (n=100) and ICNB (n=34) patients. Ninety-day high-grade complications were 20% for both cohorts. Surgical complications included urinary leak, ureteroileal stricture, wound dehiscence/abscess, adhesions, incisional hernia, and significant bleeding, which collectively comprised 39.4% (13 of 33) of 90-d major complications. Infectious complications including sepsis, wound abscess and pelvic collection comprised 36.3% (12 of 33) of 90-d major complications. There was one case of malignant ascites. There were three times as many IC as NB cases in this cohort, and IC patients had significantly more advanced histopathologic stage but had comparable complication rates to the NB cohort. Ileus rates were not significantly different between diversion types (3).
Simone et al. recently reported 180-d complications in their 45 patient cohort of ICNB. Thirty-five percent of patients experienced high-grade, 180-d complications. These included two cases of >5 cm lymphocele requiring surgical correction, five cases of hydronephrosis requiring mono/bilateral nephrostomy, one symptomatic lymphocele requiring percutaneous drainage, three bowel occlusions requiring intestinal obstruction repair, and one case of anastomosis stenosis with hydronephrosis that required ureteral reimplantation monitoring (58).
Yuh et al. reported on perioperative outcomes of RARC with ECUD (IC =62, NB =86, CCD =48). Authors reported 35% high-grade complications at 90-d with a quarter of all major complications being related to infection. High-grade complications at 90-d also included procedural (19.5%), genitourinary (14.2%) and respiratory (13.3%) complications (52). Xylinas et al. also reported on perioperative outcomes of ECUD (IC =109, NB =40, CCD =26). Authors reported a 34% 90-d complication rate with 8.6% of complications being high-grade including uretero-vesical stricture, vesico-urethral stricture, vesico-vaginal fistula, and myocardial infarction (61).
While it has been shown that RARC with ECUD affords all benefits of ORC in the elderly, no studies to date address complications of RARC with ICUD in this population (65,66).
Learning curve cases and surgical experience could be confounding factors in reporting of complication rates; however, incorporation of these cases minimizes selection bias and may be the best assessment of true complication rates before larger series are available.
Blood transfusion
Blood transfusion rates have been shown to increase mortality and complication rate after robotic cystectomy (3). In a large study of ORC by Morgan et al., in which 41.6% of patients received a perioperative blood transfusion, patients had significantly worse probability of overall survival at three years with increasing units of blood transfused (67). In a smaller study of RARC and ORC, where all urinary diversions were performed extracorporeally, it was found that while transfusion rates were nearly doubled for the ORC population, survival rates remained the same between the two cohorts (68). Similar results were found when comparing a large ICUD vs. ECUD population, with patients undergoing ICUD receiving blood transfusions significantly less frequently (13). Transfusion rates were as low as 10.0% and 4.5% for the largest series of ICIC and ICNB, respectively, with survival rates between 70–85% (21,30).
Deep vein thrombosis
Perioperative incidence of DVT following ORC has been estimated between 5–6% (2,23,64,69). Level 1 evidence supports the utility of antithrombotic treatment following radical cystectomy (70). The range of rates in the ICUD literature is about 2–3%. More detailed reports of DVT with larger cohorts are desired (21,29).
Stricture
Anastomotic stricture is an important cause of renal functional decline in this population, and can vary by length of postoperative follow-up and operative approach (71). Strictures were found to be more common in men, those with organ-confined disease, and in patients who were node positive (72). Stricture rates from the RAZOR trial were 7% vs. 9% for ORC and RARC, respectively (12). Retrospective series comparing RARC and ORC showed rates of stricture between 8.5–12.0% (72,73). In an ORC cohort, prior abdominal surgery was shown to be a strong predictor of uretero-enteric stricture development in 10 years when compared to patients without prior abdominal surgery (74).
It has been postulated that ICUD is consistent with lower stricture rates, as it requires shorter ureteric length than ECUD, avoiding distal ischemia and subsequent stricture. On the contrary, stricture rates for ICUD and ECUD were found to be 3.8–8.3% (14,30,56,57) and 6.3–7.4% (14,52,61), respectively. Supporting this, a recent study of 440 patients with a median follow-up of 23 months reported that patients who underwent ICUD had significantly greater odds (OR, 3.28) of developing stricture (75). Evaluating post-operative renal function, Tan et al. used upper tract CT imaging as a surrogate marker of low-pressure storage in their 20 patients NB cohort. At median follow-up of 21.5 months, 18 (90%) patients showed no hydronephrosis while 2 (10%) patients showed mild hydronephrosis. In 12 (60%) patients, there was no significant change in renal function post-operatively. One (5%) patient was reported to have an EGFR decline of 34.5% after AC, and 5 (20%) patients had a decline of 16.8% from baseline, three of which had previous renal impairment (56).
Ileus
Patients undergoing radical cystectomy are at significant risk for developing postoperative ileus, which becomes an important determinant of LOS, readmission and possibly longer-term complications. Patients undergoing RARC are expected to have lower rates of ileus than those undergoing ORC due to intracorporeal extirpation, reduced perioperative fluid shifts, and reduced bowel handling; however, a reliable comparison is difficult due to discrepancies between surgeon experience and patient comorbidities (2,18). While originally implemented by colorectal surgeons, ERAS protocols for radical cystectomy have decreased rates of ileus as well as hospital costs for all approaches, especially in settings where Alvimopan is used (76-78).
NAC and AC
In an ORC cohort assessing perioperative outcomes in those receiving NAC and radical cystectomy vs. those treated with radical cystectomy alone, no differences were observed in readmission or complication rates between the two groups (79). A recent study by Tan et al. comparing ICIC and ICNB patients showed that patients who received NAC were not significantly more likely to experience 90-d major complications (3). Conversely, Nazmy et al. reported a 29.7% NAC rate for their ECNB population, and NAC was a predictor of 90-d major complications (59).
AC has been shown to be a predictor of recurrence free survival (80,81). Rates of AC in ICIC and ICNB cohorts that did not receive NAC were 18–21% and 11–19%, respectively (82). In an open vs. robotic cohort (RAZOR) of primarily ECIC diversions, robotic patients received AC more frequently (17% vs. 11%), sooner following surgery (6.7 vs. 8.8 weeks) as well as had higher rates of progression free survival than the open cohort (12).
BMI
Not surprisingly, BMI has been shown to be associated with higher readmission rates following RARC. Still, ICUD in obese patients is shown to be safe and feasible (63). Poch et al. conducted a study of 56 consecutive RARCs with ICIC with a 5-month follow-up in which 75% of patients were overweight or obese. Only EBL and early complication rates were found to be significantly higher in obese when compared to normal and overweight groups. The highest BMI recorded was 47 kg/m2. Readmissions were recorded at 30-days and not found to be significant between groups (27). Ahamadi et al. conducted a similar study in 2017 with a 13-month follow-up in which 38.4%, 33.8%, 15.7%, and 12.0% of patients were classified as normal (≤25 kg/m2), pre-obese (25–29.9 kg/m2), obese class I (30–34.9 kg/m2), and obese class II (≥35 kg/m2), respectively. Significant differences between the four groups were found in ASA score, EBL and genitourinary complications. When comparing the lowest to the highest BMI patients, there were no significant differences in the ICNB group but OTs were longer for obese patients in the ICIC group. Additionally, LOS, readmission and complication rates were similar between extremes of BMI (83).
Continence and potency
All studies reported here defined continence as 0–1 pad per day and incontinence as ≥2 pads per day. Tan et al. reported 3-month daytime and nighttime continence rates of 95% and 65%, respectively, in their ICNB cohort. Similarly, at 6 months, daytime, nighttime and overall continence rates were relatively unchanged (30,56). At 12 months, Jonsson et al. reported daytime and nighttime continence rates of 97% and 83%, respectively, in their ICNB cohort (54). In studies with longer follow-up, Schwetner et al. reported lower daytime and nighttime continence rates of 88% and 51%, respectively, at an average follow-up of 37.3 months (57). Simone et al. reported 2-year daytime and nighttime continence rates of 73% and 55.5%, respectively (58). These rates are similar to continence rates in large open series (44) and bladder cancer index (BCI) scores were not significantly different between the robotic and open approach (42). One study exists on urodynamic outcomes in NB patients but large series of systematic data are lacking.
Potency in younger cohorts has been reported and all studies presented here define potency as IIEF-5 ≥17, or ability to perform intercourse with or without PDE-5 inhibitors. Jonsson et al. reported potency in 80% (16/20) of male patients who underwent ICNB and bilateral nerve-sparing surgery with mean IIEF score of 19 (SD, 2.9) (54). Desai et al. reported potency in 81% (33/41) of men who underwent ICNB and nerve-sparing surgery (30). Tyritzis et al. also report 80% potency in ICNB nerve-spared patients at 12-months (29). Schwentner et al. report spontaneous erections in 77% (27/50) male patients at 12 months (57).
Overall, assessment of continence and potency is difficult due to limited follow-up for this metric, potentially increasing the success rate.
Learning curve
Surgeons must overcome two learning curves when considering RARC for radical cystectomy. The first is the learning curve for transition from ORC to RARC for the extirpative portion of the procedure (17). The second transition is from ECUD to ICUD for the urinary diversion (14,16,43). Once a transition has been made to RARC with ICUD, technical modifications may be implemented to improve peri- and postoperative surgical outcomes (33).
In 2018, Simone et al. reported on their early experience of 45 consecutive patients who underwent ICNB. For assessment of learning curve outcomes, the cohort was split into three groups of 15. There were significant decreases in operative and console times. Mean [range] OTs in the first and last tertiles were 370 [210–630] and 307 [220–370] minutes, respectively, indicating progressive experience narrowed the range of times with a small improvement in the mean OT. Surgeons attributed flattening of the learning curve to standardized use of staplers to configure the NB neck and posterior left aspect of the NB. There were no significant differences in transfusion rates between tertiles (58).
In 2017, Tan and colleagues reported on learning curve outcomes in their institution’s transition to ICIC. Operative times improved in the last 30 cases when compared to the first 30 cases. Mean [interquartile range (IQR)] OTs for the first and last 30 cases were 360 [330–390] and 300 [270–360] minutes, respectively. When comparing the sequential final cases of ECIC to the first cases of ICIC, there were no demographic or oncological differences between groups and OT, EBL and 30-d complication rates were improved (14).
Similarly, Azzouni et al. reported significantly decreased OTs over 100 cases of ICIC and state technical modifications to achieve this. Among these modifications were cessation of both isolating the proximal staple line and irrigating the conduit after first 25–30 cases. Notably, there were no conduit stones in the entire cohort. Early and late major complications decreased significantly in the last quartile when compared to the first, while readmission rates increased but did not reach significance (21).
Collins et al. compared learning curve outcomes of two surgeons at the same institution, one surgeon having performed 47 ICNB and the second having performed 20. The only significantly different operative outcome between the two surgeons was EBL and this measure was not rectified by increased experience for either surgeon. For the more experienced surgeon, late complication rates decreased from 50% in the first 10 patients to 0% in the last 10 patients. In the very early learning curve for this group, in 2003, there were six patients who received either no or limited PLND. Lymph node yield did not increase with experience by either surgeon (19).
Abreu et al. discussed techniques for ICUD in 103 (IC =57, NB =46) consecutive patients in which they achieved a significantly shorter LOS in both IC (11 vs. 6 days) and NB (13 vs. 7.5 days) groups when comparing the first tertile with the last tertile of patients (84).
Hayn et al. report on pathologic outcomes in a cohort of 496 patients who underwent RARC with ECUD. Authors estimated 8, 20 and 30 cases were required to achieve a lymph node yield of 12, 16 and 20, respectively. Additionally, positive surgical margin rates of <5% were achieved after surgeons’ 30th case. Less impressive, relative to the data on ICUD reported here, was the observation that 21 patients were required to reach an OT of 6.5 hours (17).
Learning curve outcomes are complicated by case complexity and certain operative factors. It is recommended that surgeons making the switch from ECUD to ICUD consider starting with ICIC before progressing to continent diversion to decrease peri- and postoperative complications (7,33).
ERP
ERP have been shown to improve postoperative recovery, reduce complications and decrease health care costs in large ORC cohorts (85,86). Recently, the European Association of Urology (EAU) Robotic Urology Section (ERUS) Scientific Working Group formulated a consensus view on proper ERP for RARC, and compared this with existing ERP recommendations for ORC and current ERAS society guidelines (87,88).
Recent publications using ERP in RARC with ICUD do not note improved 90-d complications or rates of ileus. Collins et al. showed significant improvements in LOS (9 vs. 8 days) for both IC and NB patients as well as improvements in 30-d high-grade complication rates (18% vs. 34%) for IC patients after implementation of ERP. Notably, the cohort receiving ERP was older and had higher clinical staging. Authors reported using oxycodone hydrochloride and naloxone hydrochloride dehydrate (Targiniq®, Purdue Pharmaceuticals) to reduce incidence of postoperative ileus secondary to effects of opiates, but do not comment on outcomes of this (89).
While retrospective studies of ERP have provided evidence of decreased costs and improved complication rates in the RARC population, there is still a pressing need for randomized clinical trials evaluating perioperative management of RARC patients following ERP (90).
Cost effectiveness
As RARC is rapidly adapted in centers across the world, there is debate as to whether outcomes are justified by a procedure that has markedly increased costs. In one of the first studies to assess quality of life and cost effectiveness in the RARC population, MD Anderson Cancer Center used a propensity-matched cohort to compare cost-effectiveness of RARC with ICUD (n=100) vs. ORC (n=96). While fixed cost of RARC remained nearly $20,000 more expensive than ORC over 90-d days, on incremental cost-effectiveness ratio, RARC was shown to be $2,969 less expensive per quality-adjusted life years (QALYs). Additionally, RARC was associated with a 0.32 increase of QALYs. While this cost analysis is promising, sensitivity analyses showed that all data were contingent upon RARC having significantly fewer complications and transfusions. Notably, findings applied only to ICUD as this was the primary urinary diversion used in this cohort (91).
In 2011, Lee et al. performed a similar study comparing RARC with ECUD (n=83) vs. ORC (n=103) and economics were specified by combinations of diversion and surgical type. While this study found that RARC significantly reduced LOS, decreasing LOS did not rectify the higher upfront cost of RARC utility and management. Using the Medicare Resource Based Relative Value Scale (RBRVS), authors analyzed direct and indirect costs. Direct costs included surgeon fees, per-case cost of robot and robot utility, anesthesia costs and LOS costs. Indirect cost included cost of complications. While RARC was more costly in direct costs for all diversion types, ORC was more expensive for indirect cost for IC and CCD, but not for NB. Overall cost effectiveness favoring robotics was only greater for IC patients (92).
Conclusions and future directions
Early data on RARC with ICUD support the safety and feasibility of this procedure, demonstrating non-inferior perioperative and oncologic outcomes. The increased ergonomic ability for surgeons, as well as decreased blood losses and perioperative complications for patients, may facilitate a quicker recovery period for the patient and fewer readmission rates for hospitals. These advantages, together with improved standardization and experience with the challenging technical aspects of the procedure as well as improved NAC and AC regimens, could yield more compelling outcomes in the future. The current literature varies by surgeon experience and cohort size, highlighting the need for randomized clinical trials comparing RARC with ICUD vs. RARC with ECUD. The intracorporeal RARC (iRARC) clinical trial comparing RARC with ICUD vs. ORC is in recruitment and results may shed light on this highly debated topic (93).
Acknowledgments
Amanda L. LeSueur, PhD: writing and language editing assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marc C. Smaldone and Jeffrey J. Tomaszewski) for the series “Controversies in Minimally Invasive Urologic Oncology” published in Translational Andrology and Urology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.09.45). The series “Controversies in Minimally Invasive Urologic Oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Hemelrijck M, Thorstenson A, Smith P, et al. Risk of in-hospital complications after radical cystectomy for urinary bladder carcinoma: population-based follow-up study of 7608 patients. BJU Int 2013;112:1113-20. [Crossref] [PubMed]
- Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164-74. [Crossref] [PubMed]
- Tan WS, Lamb BW, Tan MY, et al. In-depth critical analysis of complications following robot-assisted radical cystectomy with intracorporeal urinary diversion. Eur Urol Focus 2017;3:273-9. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Leow JJ, Reese SW, Jiang W, et al. Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol 2014;66:569-76. [Crossref] [PubMed]
- Parsons JK, Palazzi K, Chang D, et al. Patient safety and the diffusion of surgical innovations: a national analysis of laparoscopic partial nephrectomy. Surg Endosc 2013;27:1674-80. [Crossref] [PubMed]
- Wilson TG, Guru K, Rosen RC, et al. RARC pasadena consensus panel-review best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the pasadena consensus panel. Eur Urol 2015;67:363-75. [Crossref] [PubMed]
- Tan WS, Sridhar A, Ellis G, et al. Analysis of open and intracorporeal robotic assisted radical cystectomy shows no significant difference in recurrence patterns and oncological outcomes. Urol Oncol 2016;34:257.e1-9. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol 2015;67:1042-50. [Crossref] [PubMed]
- Khan MS, Gan C, Ahmed K, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol 2016;69:613-21. [Crossref] [PubMed]
- Nix J, Smith A, Kurpad R, et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol 2010;57:196-201. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Hussein AA, May PR, Jing Z, et al. Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. J Urol 2018;199:1302-11. [Crossref] [PubMed]
- Tan TW, Nair R, Saad S, et al. Safe transition from extracorporeal to intracorporeal urinary diversion following robot-assisted cystectomy: a recipe for reducing operative time, blood loss and complication rates. World J Urol 2019;37:367-72. [Crossref] [PubMed]
- Canda AE, Atmaca AF, Altinova S, et al. Robot-assisted nerve-sparing radical cystectomy with bilateral extended pelvic lymph node dissection (PLND) and intracorporeal urinary diversion for bladder cancer: initial experience in 27 cases. BJU Int 2012;110:434-44. [Crossref] [PubMed]
- Guru K, Seixas-Mikelus SA, Hussain A, et al. Robot-assisted intracorporeal ileal conduit: marionette technique and initial experience at Roswell park cancer institute. Urology 2010;76:866-71. [Crossref] [PubMed]
- Hayn MH, Hussain A, Mansour AM, et al. Platinum priority-bladder cancer the learning curve of robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. Eur Urol 2010;58:197-202. [Crossref] [PubMed]
- Ahmed K, Khan SA, Hayn MH, et al. Platinum priority-bladder cancer analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. Eur Urol 2014;65:340-7. [Crossref] [PubMed]
- Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder - what is the effect of the learning curve on outcomes? BJU Int 2014;113:100-7. [Crossref] [PubMed]
- Lenfant L, Verhoest G, Campi R, et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional French study. World J Urol 2018;36:1711-8. [Crossref] [PubMed]
- Azzouni FS, Din R, Rehman S, et al. The first 100 consecutive, robot-assisted, intracorporeal ileal conduits: evolution of technique and 90-day outcomes. Eur Urol 2013;63:637-43. [Crossref] [PubMed]
- Kang SG, Ko YH, Jang HA, et al. Initial Experience of robot-assisted radical cystectomy with total intracorporeal urinary diversion: comparison with extracorporeal method. J Laparoendosc Adv Surg Tech A 2012;22:456-62. [Crossref] [PubMed]
- Pruthi RS, Nix J, Mcrackan D, et al. Robotic-assisted laparoscopic intracorporeal urinary diversion. Eur Urol 2010;57:1013-21. [Crossref] [PubMed]
- Goh AC, Gill IS, Lee DJ, et al. Robotic intracorporeal orthotopic ileal neobladder: replicating open surgical principles. Eur Urol 2012;62:891-901. [Crossref] [PubMed]
- Soria F, Moschini M, D'andrea D, et al. Comparative effectiveness in perioperative outcomes of robotic versus open radical cystectomy: results from a multicenter contemporary retrospective cohort study. Eur Urol Focus 2020;6:1233-9. [Crossref] [PubMed]
- Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol 2003;44:337-9. [Crossref] [PubMed]
- Poch MA, Stegemann A, Chandrasekhar R, et al. Does body mass index impact the performance of robot-assisted intracorporeal ileal conduit? J Endourol 2012;26:857-60. [Crossref] [PubMed]
- Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy: description of an evolved approach to radical cystectomy. Eur Urol 2013;64:654-63. [Crossref] [PubMed]
- Tyritzis SI, Hosseini A, Collins J, et al. Platinum priority-bladder cancer oncologic, functional, and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion. Eur Urol 2013;64:734-41. [Crossref] [PubMed]
- Desai MM, Gill IS, Luis de Castro Abreu A, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol 2014;192:1734-40. [Crossref] [PubMed]
- Novara G, Catto JWF, Wilson T, et al. RARC Pasadena consensus panel-review systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol 2015;67:376-401. [Crossref] [PubMed]
- Chan KG, Guru K, Wiklund P, et al. RARC Pasadena consensus panel-surgery in motion robot-assisted radical cystectomy and urinary diversion: technical recommendations from the pasadena consensus panel. Eur Urol 2015;67:423-31. [Crossref] [PubMed]
- Desai MM, de Abreu ALC, Goh AC, et al. Robotic intracorporeal urinary diversion: technical details to improve time efficiency. J Endourol 2014;28:1320-7. [Crossref] [PubMed]
- Liu L, Chen M, Li Y, et al. Technique selection of bricker or wallace ureteroileal anastomosis in ileal conduit urinary diversion: a strategy based on patient characteristics. Ann Surg Oncol 2014;21:2808-12. [Crossref] [PubMed]
- Hussein AA, Ahmed YE, Kozlowski JD, et al. Robot-assisted approach to ‘W’-configuration urinary diversion: a step-by-step technique. BJU Int 2017;120:152-7. [Crossref] [PubMed]
- Bishop CV, Vasdev N, Boustead G, et al. Robotic intracorporeal ileal conduit formation: initial experience from a single UK centre. Adv Urol 2013;2013:642836 [Crossref] [PubMed]
- Kouba E, Sands M, Lentz A, et al. A comparison of the Bricker versus Wallace ureteroileal anastomosis in patients undergoing urinary diversion for bladder cancer. J Urol 2007;178:945-8; discussion 948-9. [Crossref] [PubMed]
- Evangelidis A, Lee EK, Karellas ME, et al. Evaluation of ureterointestinal anastomosis: Wallace vs Bricker. J Urol 2006;175:1755-8; discussion 1758. [Crossref] [PubMed]
- Kang SG, Kang SH, Lee YG, et al. Robot-assisted radical cystectomy and pelvic lymph node dissection: a multi-institutional study from Korea. J Endourol 2010;24:1435-40. [Crossref] [PubMed]
- Desai MM, Simone G, de Castro Abreu AL, et al. Robotic intracorporeal continent cutaneous diversion. J Urol 2017;198:436-44. [Crossref] [PubMed]
- Wiklund NP, Poulakis V. Robotic neobladder. BJU Int 2011;107:1514-37. [Crossref] [PubMed]
- Satkunasivam R, Santomauro M, Chopra S, et al. Platinum priority-bladder cancer robotic intracorporeal orthotopic neobladder: urodynamic outcomes, urinary function, and health-related quality of life. Eur Urol 2016;69:247-53. [Crossref] [PubMed]
- Pyun JH, Kim HK, Cho S, et al. Robot-assisted radical cystectomy with total intracorporeal urinary diversion: comparative analysis with extracorporeal urinary diversion. J Laparoendosc Adv Surg Tech A 2016;26:349-55. [Crossref] [PubMed]
- Hautmann RE, de Petriconi R, Gottfried HW, et al. The ileal neobladder: complications and functional results in 363 patients after 11 years of followup. J Urol 1999;161:422-7; discussion 427-8. [Crossref] [PubMed]
- Studer UE, Zingg EJ. Ileal orthotopic bladder substitutes. What we have learned from 12 years' experience with 200 patients. Urol Clin North Am 1997;24:781-93. [Crossref] [PubMed]
- Sathianathen NJ, Kalapara A, Frydenberg M, et al. Robotic assisted radical cystectomy vs open radical cystectomy: systematic review and meta-analysis. J Urol 2019;201:715-20. [Crossref] [PubMed]
- Raza SJ, Wilson T, Peabody JO, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. Eur Urol 2015;68:721-8. [Crossref] [PubMed]
- Collins JW, Hosseini A, Adding C, et al. Early recurrence patterns following totally intracorporeal robot-assisted radical cystectomy: results from the EAU robotic urology section (ERUS) scientific working group. Eur Urol 2017;71:723-6. [Crossref] [PubMed]
- Nguyen DP, Al B, Al Awamlh H, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer HHS public access intervention-ORC and RARC. Eur Urol 2015;68:399-405. [Crossref] [PubMed]
- Hussein AA, Saar M, May PR, et al. Early oncologic failure after robot-assisted radical cystectomy: results from the international robotic cystectomy consortium. J Urol 2017;197:1427-36. [Crossref] [PubMed]
- Goh AC, Aghazadeh MA, Krasnow RE, et al. Robotic intracorporeal continent cutaneous urinary diversion: primary description. J Endourol 2015;29:1217-20. [Crossref] [PubMed]
- Yuh BE, Nazmy M, Ruel NH, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol 2012;62:806-13. [Crossref] [PubMed]
- Marshall SJ, Hayn MH, Stegemann AP, et al. Impact of surgeon and volume on extended lymphadenectomy at the time of robot-assisted radical cystectomy: results from the international robotic cystectomy consortium (IRCC). BJU Int 2013;111:1075-80. [Crossref] [PubMed]
- Jonsson MN, Adding LC, Hosseini A, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur Urol 2011;60:1066-73. [Crossref] [PubMed]
- Khan MS, Elhage O, Challacombe B, et al. Analysis of early complications of robotic-assisted radical cystectomy using a standardized reporting system. Urology 2011;77:357-62. [Crossref] [PubMed]
- Tan WS, Sridhar A, Goldstraw M, et al. Robot-assisted intracorporeal pyramid neobladder. BJU Int 2015;116:771-9. [Crossref] [PubMed]
- Schwentner C, Sim A, Balbay MD, et al. Robot-assisted radical cystectomy and intracorporeal neobladder formation: on the way to a standardized procedure. World J Surg Oncol 2015;13:3. [Crossref] [PubMed]
- Simone G, Papalia R, Misuraca L, et al. Robotic intracorporeal padua ileal bladder: surgical technique, perioperative, oncologic and functional outcomes. Eur Urol 2018;73:934-40. [Crossref] [PubMed]
- Nazmy M, Yuh B, Kawachi M, et al. Trauma/reconstruction/diversion early and late complications of robot-assisted radical cystectomy: a standardized analysis by urinary diversion type. J Urol 2014;191:681-7. [Crossref] [PubMed]
- Torrey RR, Chan KG, Yip W, et al. Laparoscopy and robotics functional outcomes and complications in patients with bladder cancer undergoing robotic-assisted radical cystectomy with extracorporeal indiana pouch continent cutaneous urinary diversion. Urology 2012;79:1073-8. [Crossref] [PubMed]
- Xylinas E, Green DA, Otto B, et al. Robotic-assisted radical cystectomy with extracorporeal urinary diversion for urothelial carcinoma of the bladder: analysis of complications and oncologic outcomes in 175 patients with a median follow-up of 3 years. Urology 2013;82:1323-9. [Crossref] [PubMed]
- Styn NR, Montgomery JS, Wood DP, et al. Matched comparison of robotic-assisted and open radical cystectomy. Urology 2012;79:1303-8. [Crossref] [PubMed]
- Al-Daghmin A, Aboumohamed A, Din R, et al. Laparoscopy and robotics readmission after robot-assisted radical cystectomy: outcomes and predictors at 90-day follow-up. Urology 2014;83:350-6. [Crossref] [PubMed]
- Stimson CJ, Chang SS, Barocas DA, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol 2010;184:1296-300. [Crossref] [PubMed]
- Coward RM, Smith A, Raynor M, et al. Feasibility and outcomes of robotic-assisted laparoscopic radical cystectomy for bladder cancer in older patients. Urology 2011;77:1111-4. [Crossref] [PubMed]
- Guillotreau J, Miocinovic R, Gamé X, et al. Outcomes of laparoscopic and robotic radical cystectomy in the elderly patients. Urology 2012;79:585-90. [Crossref] [PubMed]
- Morgan TM, Barocas DA, Chang SS, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol 2013;31:871-7. [Crossref] [PubMed]
- Nepple KG, Strope SA, Grubb RL, et al. Early oncologic outcomes of robotic versus open radical cystectomy for urothelial cancer. Urol Oncol 2013;31:894-8. [Crossref] [PubMed]
- VanDlac AA, Cowan NG, Chen Y, et al. Timing, incidence and risk factors for venous thromboembolism in patients undergoing radical cystectomy for malignancy: a case for extended duration pharmacological prophylaxis. J Urol 2014;191:943-7. [Crossref] [PubMed]
- Kakkar VV, Balibrea JL, Martinez-Gonzalez J, et al. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223-9. [Crossref] [PubMed]
- Hautmann RE, De Petriconi RC, Volkmer BG. 25 years of experience with 1,000 neobladders: long-term complications. J Urol 2011;185:2207-12. [Crossref] [PubMed]
- Richards KA, Cohn JA, Large MC, et al. The effect of length of ureteral resection on benign ureterointestinal stricture rate in ileal conduit or ileal neobladder urinary diversion following radical cystectomy. Urol Oncol 2015;33:65.e1-8. [Crossref] [PubMed]
- Anderson CB, Morgan TM, Kappa S, et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter? J Urol 2013;189:541-7. [Crossref] [PubMed]
- Katherine AA, Emily AV, Gillian S, et al. Predictors of benign uretero-enteric anastomotic strictures after radical cystectomy and urinary diversion. Urology 2018; [Epub ahead of print].
- Ahmed YE, Hussein AA, May PR, et al. Natural history, predictors and management of ureteroenteric strictures after robot assisted radical cystectomy. J Urol 2017;198:567-74. [Crossref] [PubMed]
- Packiam VT, Agrawal VA, Pariser JJ, et al. Redefining the implications of nasogastric tube placement following radical cystectomy in the alvimopan era. World J Urol 2017;35:625-31. [Crossref] [PubMed]
- Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology 2018;111:86-91. [Crossref] [PubMed]
- Lee CT, Chang SS, Kamat AM, et al. Platinum priority-bladder cancer alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol 2014;66:265-72. [Crossref] [PubMed]
- Gandaglia G, Popa I, Abdollah F, et al. The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy: a population-based study. Eur Urol 2014;66:561-8. [Crossref] [PubMed]
- Raza SJ, Tawfeeq M, Al-Daghmin A, et al. Robot-assisted intracorporeal urinary diversion: where do we stand in 2014? Urol Clin North Am 2014;41:503-9. [Crossref] [PubMed]
- Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol 2016;34:825-32. [Crossref] [PubMed]
- Hussein AA, Hinata N, Khan N, et al. Does robot-assisted approach to radical cystectomy influence surgeon choice for urinary diversion? J Urol Nephrol Open Access 2016;2:1-5.
- Ahmadi N, Clifford TG, Miranda G, et al. Impact of body mass index on robot-assisted radical cystectomy with intracorporeal urinary diversion. BJU Int 2017;120:689-94. [Crossref] [PubMed]
- Abreu AL, Chopra S, Azhar RA, et al. Robotic radical cystectomy and intracorporeal urinary diversion: the USC technique. Indian J Urol 2014;30:300-6. [Crossref] [PubMed]
- Pruthi RS, Nielsen M, Smith A, et al. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg 2010;210:93-9. [Crossref] [PubMed]
- Bazargani ST, Djaladat H, Ahmadi H, et al. Gastrointestinal complications following radical cystectomy using enhanced recovery protocol. Eur Urol Focus 2018;4:889-94. [Crossref] [PubMed]
- Collins JW, Patel H, Adding C, et al. Enhanced recovery after robot-assisted radical cystectomy: EAU robotic urology section scientific working group consensus view. Eur Urol 2016;70:649-60. [Crossref] [PubMed]
- Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (ERAS®) society recommendations. Clin Nutr 2013;32:879-87. [Crossref] [PubMed]
- Collins JW, Adding C, Hosseini A, et al. Scandinavian Journal of Urology Introducing an enhanced recovery programme to an established totally intracorporeal robot-assisted radical cystectomy service Introducing an enhanced recovery programme to an established totally intracorporeal robot-assiste. Scand J Urol 2016;50:39-46. [Crossref] [PubMed]
- Azhar RA, Bochner B, Catto J, et al. Enhanced recovery after urological surgery: a contemporary systematic review of outcomes, key elements, and research needs. Eur Urol 2016;70:176-87. [Crossref] [PubMed]
- Kukreja JB, Metcalfe MJ, Qiao W, et al. Cost-effectiveness of robot-assisted radical cystectomy using a propensity-matched cohort. Eur Urol Focus 2020;6:88-94. [Crossref] [PubMed]
- Lee R, Ng CK, Shariat SF, et al. The economics of robotic cystectomy: cost comparison of open versus robotic cystectomy. BJU Int 2011;108:1886-92. [Crossref] [PubMed]
- Catto JWF, Khetrapal P, Ambler G, et al. Multidomain quantitative recovery following radical cystectomy for patients within the robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy randomised controlled trial: the first 30 patients. Eur Urol 2018;74:531-4. [Crossref] [PubMed]



