
Prevalence, clinicopathological features, and prognosis in upper tract urinary carcinoma patients with severe preoperative chronic kidney disease
Introduction
Upper tract urinary carcinoma (UTUC) is any carcinoma that arises from the urothelium of the urinary tract from the renal pelvis to the distal ureter. UTUC is relatively rare, with an approximate annual incidence of 1–2 cases per 100,000, and it accounts for only 5% to 10% of all urothelial carcinomas (1,2). However, UTUC has a high incidence in China and is often complicated by chronic kidney disease (CKD), possibly related to exposure to aristolochic acids (AA) (3,4). It is generally acknowledged that radical nephroureterectomy (RNU) with excision of the bladder cuff is the standard procedure for the patients with UTUC (5-7). Nevertheless, chances are that kidney function is compromised due to solitary kidney, and renal replacement therapy (kidney transplant or dialysis) is the inevitable outcome for certain patients.
Only a few studies have reported the prevalence and factors associated with baseline kidney function in UTUC patients because of the low prevalence of UTUC. It was reported that the prevalence of preoperative CKD was much higher in UTUC patients than in the normal Chinese population and patients with end-stage renal disease had a higher risk for UTUC, and the tumor stage and tumor grade of UTUC increased with the severity preoperative CKD stage (8). However, few articles focused on the population of patients with severe preoperative CKD (eGFR <30 mL/min), which faces a much higher probability of renal replacement therapy due to the decline in renal function after surgery. Therefore, we conducted the present study to investigate the prevalence, clinicopathological features, and prognosis in UTUC patients with severe preoperative CKD.
Methods
Patient enrollment and evaluation
Between January 2002 and December 2011, 883 consecutive patients diagnosed histologically with UTUC received surgical treatment in the Department of Urology, Peking University First Hospital. One hundred and fifty-two patients were excluded from this study because of missing follow-up data (n=74); accompanying other malignancies (n=6); receiving other treatment than RNU (n=72). Finally, 731 patients were enrolled. All patients underwent standard RNU with bladder cuff resection through an extravesical approach. In the extravesical technique, the intramural portion of the ureter was completely dissected. With gentle traction on the ureter, a right-angle clamp or stapler was used to transect the distal ureter with its bladder cuff. The bladder was also closed with a two-layer suture. Routine lymph node dissection was performed when enlarged lymph nodes were found by preoperative imaging or intraoperative observation. The status of those patient who did not received lymph node dissection was defined as pNx.
eGFR was calculated using re-expressed Modification of Diet in Renal Disease (MDRD) formulas modified based on the Chinese population [eGFR (mL/min/1.73 m2) =175 × Scr-1.234 × age-0.179 (×0.79 if female)] (9). CKD stage was determined by the criterion provided by the American National Kidney Foundation. Severe preoperative CKD was defined as CKD stage 4–5 (eGFR <30 mL/min). Exposure to AA was defined as a history of long-term exposure (>3 months) of intermittent intake of regular doses of AA-containing traditional Chinese medicine. Staging was assessed according to the 2002 Union for International Cancer Control (UICC) TNM classification guidelines. Tumor grade was determined by the Word Health Organization (WHO) classification of 1973. Tumor multifocality was defined as the synchronous presence of two or more pathologically confirmed macroscopic tumors in the upper tract. Bilateral UTUC was defined as bilateral urothelial carcinoma on preoperative imaging, and the imaging result was confirmed using pathology. The clinical and histological characteristics were reviewed and collected from an electronic database of the patients’ medical records.
For patients who were followed at our institute, the follow-up regimen included cystoscopy every 3 months for the first 3 years. The cystoscopy intervals were extended to 1 year thereafter. Chest X-ray, urine cytology, serum creatinine test, and abdominal ultrasound or CT/MRI were performed at the same time.
New contralateral was defined as urothelial carcinoma in the contralateral upper urinary tract that was confirmed by pathology or positive urine cytology plus direct visualization of the tumors by endoscopy or conclusive imaging study. Intravesical recurrence (IVR) was defined as finding a subsequent bladder tumor during cystoscopy, and it was confirmed by pathology (10). Cancer-specific survival (CSS), contralateral recurrence-free survival and IVR-free survival were determined at the last follow-up based on the examination results. Overall survival (OS) was determined by a review of the patients’ medical records and from the Chinese National Statistical Office database.
Statistical analysis
All the data were analyzed by SPSS 22.0 (IBM Corp, Armonk, NY, USA). Pearson’s test and the Chi-square test were used to determine the distribution of categorical variables, and the Mann-Whitney U test was used for continuous variables. Multivariate logistic regression was used to calculate the predictive factors. Univariate analysis using the log-rank test and multivariate analysis using Cox’s proportional hazard regression model were used. Only those variables that were identified as significant in the univariate analysis were included in the multivariate analysis. All reported P values were 2 sided with statistical significance considered at P<0.05.
Results
Clinical and histological characteristics
The patients included 319 (43.6%) men and 412 (56.4%) women with an average age of 66 [20–90] years [a median age of 69 (IQR 60–75)] at the time of surgery. In all, 73 patients suffered from severe CKD (eGFR <30 mL/min), while 658 patients had CKD stage 3 or less (eGFR ≥30 mL/min). Forty-seven (64.4%) of the patients with severe CKD suffered from renal function decrease with a median decreased eGFR of 2.31 (IQR 0.90–4.90) mL/min and 54 (74.0%) of them underwent dialysis after surgery. In our cohort, 98 (13.4%) patients received lymph node dissection with a median lymph node number of 3 (IQR 2–6) and a mean number of 4.6 (range, 0–31). 50 of them were pN+ with a median positive lymph node number of 1 (IQR 0–1) and a mean number of 1.4 (range, 0–23). The status of those patient who did not received lymph node dissection was defined as pNx. The ratio of pNx vs. pN+ was 633 vs. 50.
The patient clinical and histological data, stratified according to the CKD stage, are shown in Table 1 and Table S1. The univariate analysis indicated that patients with severe CKD tended to be female (P=0.007) and to have lower body mass index (BMI) (P=0.003), synchronous bilateral tumors (P=0.001), concomitant bladder tumor (P<0.001), multifocal upper tract tumors (P=0.001), lower pathological T stage (P=0.038), exposure of AA (P<0.001), and tumor necrosis (P<0.001). In multivariate logistic regression, after controlling for clinical factors, female gender (OR =1.791; 95% CI: 1.018–3.150; P=0.043), lower BMI (OR =0.452; 95% CI: 0.262–0.778; P=0.004), concomitant bladder tumor (OR =2.944; 95% CI: 1.360–6.373; P=0.006), lower pathological T stage (OR =0.578; 95% CI: 0.339–0.984; P=0.043), tumor necrosis(OR =2.764; 95% CI: 1.411–5.416; P=0.003), and exposure of AA (OR =3.115; 95% CI: 1.536–6.316; P=0.002)were still significantly related to severe CKD.
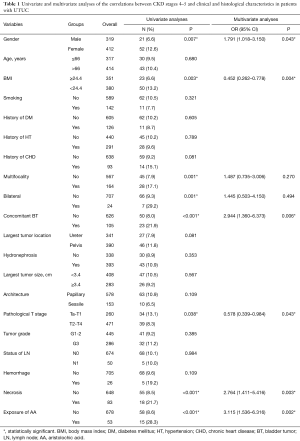
Full table
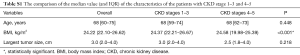
Full table
Predictive role on oncologic outcomes
The median follow-up duration of this cohort of patients was 44 (IQR 12–155) months. In all, 160 (21.9%) patients died, and 133 (18.2%) died of urothelial cancer. In the cohort of patients with severe CKD, 25 (34.2%) patient died. Seventeen (23.3%) patients died of UTUC, and 8 (11.0%) patients died from life in dialysis or other reasons. Moreover, new contralateral UTUC occurred in 41 (5.61%) cases. The 5-year OS and CSS were 83.9% and 86.2%, respectively.
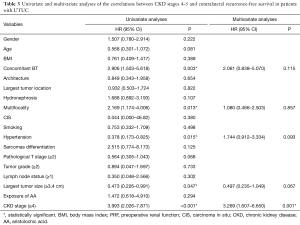
Kaplan-Meier analysis showed that severe preoperative CKD was significantly associated with worse OS (P=0.035) and worse contralateral recurrence-free survival (P<0.001), but it was not associated with CSS (P=0.386) or IVR-free survival (P=0.690), as shown in Figure 1. In multivariable analysis, in addition to severe preoperative CKD stage (HR =1.840; 95% CI: 1.150–2.944; P=0.011), female gender (HR =0.593; 95% CI: 0.428–0.822; P=0.002), concomitant bladder tumor (HR =1.883; 95% CI: 1.275–2.782; P=0.001), T stage 2–4 (HR =2.247; 95% CI: 1.433–3.524; P<0.001), lymph node metastasis (HR =1.884; 95% CI: 1.104–3.216; P=0.020) and tumor hemorrhage (HR =1.924; 95% CI: 1.020–3.628; P=0.043) were significantly associated with worse OS. And, in multivariable analysis severe preoperative CKD stage (HR =3.269; 95% CI: 1.607–6.650; P=0.001) was an independent risk factor for new contralateral UTUC (Tables 2,3).
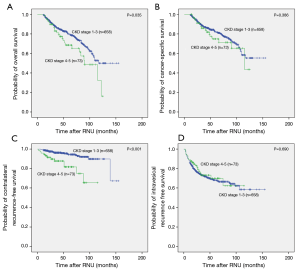
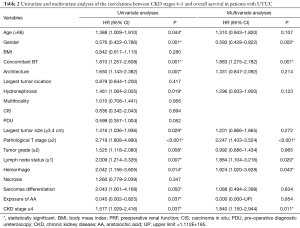
Full table
Full table
Discussion
For UTUC patients, RNU with excision of the bladder cuff is the current gold-standard treatment (5). The risk of death increased as the estimated GFR decreased below 60 mL/min/1.73 m2. The hazard ratio for death was 1.2 with an eGFR of 45 to 59 mL/min/1.73 m2, 1.8 with an eGFR of 30 to 44 mL/min/1.73 m2, 3.2 with an eGFR of 15 to 29 mL/min/1.73 m2, and 5.9 with an eGFR of less than 15 mL/min/1.73 m2 (11). Attention should be paid to CKD as an independent risk factor for comorbidity and death in UTUC patients. Previous publications from our center have indicated that lower preoperative eGFR is an independent risk factor for renal insufficiency after RNU (12). Based on a large Chinese cohort, the present study showed that female gender, lower BMI, concomitant bladder tumor, lower pathological T stage, and tumor necrosis were independently associated with severe preoperative CKD in UTUC patients, and severe preoperative CKD stage (eGFR <30 mL/min) was significantly associated with worse OS and was an independent risk factor for new contralateral UTUC.
In our research, severe preoperative CKD was related to tumor multiplicity, concomitant bladder tumor and synchronous bilateral UTUC, which has been indicated by other researchers. A previous study conducted by Novara confirmed that tumor multifocality and concomitant muscle-invasive bladder urothelial tumors were independent predictors of cancer-specific mortality in UTUC patients (13). Brown has proved that synchronous bilateral UTUC has a negative impact on disease-specific survival (14). This result indicated that complete urinary tract examination should be performed before surgery for UTUC patients with renal insufficiency, as the whole urothelial epithelium is at risk for cancer and possesses greater risks of cancer progression, and follow-up should be conducted strictly for those patients.
The present study revealed severe preoperative CKD stage was significantly related to lower tumor stage (P=0.026). Currently, the relation between preoperative CKD stage and tumor stage is controversial. Hung revealed that preoperative CKD stage was significantly related to higher tumor stage and higher tumor grade in UTUC patients (15). In contrast, a retrospective research study revealed that UTUC patients in the Balkan endemic nephropathy area from 1957–1986 had a lower tumor stage (pTa-pT1) compared to the control group (16). The present study revealed that severe preoperative CKD stage was significantly related to lower tumor stage (P=0.026). A plausible explanation might be the wide consumption of Chinese herbs containing AA by females in China with the purpose of losing weight, regulating the menstrual cycle, increasing breast milk production and reducing the secretion of leucorrhea (17,18). It has been reported that AA may act as a nephron-toxin inducing tubulointerstitial nephritis, which could lead to end-stage renal disease (19). However, aristolactam deoxyribonucleic acid (DNA) adducts and p53 mutations have been found in AA-consuming people, and Balkan endemic nephropathy as a form of AA nephropathy possessed a 100-fold increased frequency of UTUC compared with nonendemic areas, both of which proved AA as person carcinogen (17,20).
Chen et al. revealed a close relation between female patients of UTUC and AA-consumption (17). Additionally, it was reported that female gender was predominant in the Balkan endemic area in South Serbia (16). In our cohort, female gender was significantly related to severe CKD stage (P=0.007), in accordance with previous research. This result confirmed the relationship between the consumption of AA medicine and UTUC patients in our cohort, in spite of the lack of data on medicine consumption. The present study revealed that severe preoperative CKD stage was significantly related to lower tumor stage, which may support the hypothesis that AA-related UTUCs tend to have lower malignancy (18).
In the present study, severe preoperative CKD stage was significantly related to OS, but not to CSS, which may contradict previous studies. Cukuranovic discovered no difference in cumulative survival between the Balkan endemic area and a control area (16). A higher proportion of the population with reduced renal function was found in the endemic area; however, specific CKD stage at surgery was not presented, and the severity of the influence from AA was impossible to assess. Chen revealed a worse CSS rate in CKD UTUC patients (P=0.0399). Conversely, a higher ratio of high grade tumor was significantly related to CKD patients (21). However, tumor grade was not related to preoperative CKD stage in our research. This difference may be explained by the unique influence from AA-medicine in the Chinese UTUC population. This phenomenon might be another unique characteristic of AA-induced UTUC in the Chinese population.
In our cohort, new contralateral UTUC occurred in 41 (5.61%) cases, which is similar to previous studies (22-25). The reported risk factors were gender, concomitant bladder cancer, renal insufficiency and uremia. In our study, severe preoperative CKD stage was an independent risk factor for new contralateral UTUC. It is difficult to explain this outcome. The potential pathophysiologic mechanisms of recurrent urothelial tumors have been explained by two hypothesis: the monoclonality hypothesis (including intraepithelial migration and intraluminal seeding) and the field cancerization hypothesis (26,27). The hypothesis of intraluminal seeding explains only a minority of new contralateral UTUC. Because few patients suffered from vesicoureteral reflux; although some patients underwent ureteroscopy, no correlations have been shown between ureteroscopy and new contralateral UTUC. Meanwhile, the mechanism of field cancerization may be a factor in the majority of patients with new contralateral UUTC. Besides, exposure of AA could bring nephrotoxic and carcinogenic toxins to induce neoplasms of the entire urothelial field, which may illustrate why new contralateral UTUC was associated with severe preoperative CKD stage in the current study. Based on our findings, we suggest that UTUC patients with severe CKD stage who would likely require dialysis, prophylactic RNU is a reasonable choice after first RNU for unilateral UTUC.
There are certainly some limitations of this study. It was a retrospective study, subjecting it to selection and recall bias and preventing us from obtaining data on the consumption of Chinese herbs in our patients and from performing an analysis of the relationship between AA-medicine consumption and preoperative CKD stage. Furthermore, the proportion of patients with severe preoperative CKD is relatively small compared with the large cohort.
In conclusion, the data from the current study suggest that female gender, lower BMI, concomitant bladder tumor, lower pathological T stage, and tumor necrosis were independently associated with severe preoperative CKD in UTUC patients. UTUC patients with severe preoperative CKD possess worse OS and higher possibility of contralateral upper urinary tract recurrence.
Acknowledgments
The authors thank the entire staff of the Department of Urology, Peking University First Hospital. Structured data processing occurred partially using Medbanks’ approach (Medbanks Network Technology Co. Ltd., Beijing, China).
Funding: This study was funded by the National Natural Science Foundation of China [81772703], the Beijing Natural Science Foundation [L182004, 7152146, 7172219], the Clinical Features Research of Capital [No. Z151100004015173], the Capital Health Research and Development of Special [2016-1-4077], the Fund for Fostering Young Scholars of Peking University Health Science Center [BMU2017PY009] and the Collaborative Research Foundation of Peking University Health Science Center and National Taiwan University, the College of Medicine [BMU20120318].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments. Approval for this research was obtained from the internal ethics review board of Peking University First Hospital {approval No. 2015[977]} and patient consent obtained.
References
- Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol 2018;73:111-22. [Crossref] [PubMed]
- Siegel RL, Miller KD, Dvm AJ. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bara T Jr, Gurzu S, Sugimura H, et al. A systematic review of the possible carcinogenic role of the aristolochic acid. Rom J Morphol Embryol 2017;58:41-4. [PubMed]
- Stiborova M, Arlt VM, Schmeiser HH. Balkan endemic nephropathy: an update on its aetiology. Arch Toxicol 2016;90:2595-615. [Crossref] [PubMed]
- Baard J, de Bruin DM, Zondervan PJ, et al. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol 2017;14:181-91. [Crossref] [PubMed]
- Cubelli M, Di Nunno V, Rihawi K, et al. Immune checkpoint inhibitors for metastatic bladder cancer. Transl Cancer Res 2017;6:S720-32. [Crossref]
- Raman JD, Ng CK, Boorjian SA, et al. Bladder cancer after managing upper urinary tract transitional cell carcinoma: predictive factors and pathology. BJU Int 2005;96:1031-5. [Crossref] [PubMed]
- Wang SM, Lai MN, Chen PC, et al. Increased upper and lower tract urothelial carcinoma in patients with end-stage renal disease: a nationwide cohort study in Taiwan during 1997-2008. Biomed Res Int 2014;2014:149750. [PubMed]
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-44. [Crossref] [PubMed]
- Fang D, Xiong GY, Li XS, et al. Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a large Chinese center experience. J Formos Med Assoc 2014;113:820-7. [Crossref] [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [Crossref] [PubMed]
- Fang D, Zhang Q, Li X, et al. Nomogram predicting renal insufficiency after nephroureterectomy for upper tract urothelial carcinoma in the Chinese population: exclusion of ineligible candidates for adjuvant chemotherapy. Biomed Res Int 2014;2014:529186. [PubMed]
- Novara G, De Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer 2007;110:1715-22. [Crossref] [PubMed]
- Brown GA, Busby JE, Wood CG, et al. Nephroureterectomy for treating upper urinary tract transitional cell carcinoma: Time to change the treatment paradigm? BJU Int 2006;98:1176-80. [Crossref] [PubMed]
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735-40. [Crossref] [PubMed]
- Cukuranovic R, Ignjatovic I, Visnjic M, et al. Characteristics of upper urothelial carcinoma in an area of Balkan endemic nephropathy in south Serbia. A fifty-year retrospective study. Tumori 2010;96:674-9. [Crossref] [PubMed]
- Chen CH, Dickman KG, Huang CY, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. Int J Cancer 2013;133:14-20. [Crossref] [PubMed]
- Xiong G, Liu J, Tang Q, et al. Prognostic and predictive value of epigenetic biomarkers and clinical factors in upper tract urothelial carcinoma. Epigenomics 2015;7:733-44. [Crossref] [PubMed]
- Hung PH, Shen CH, Chiu YL, et al. The aggressiveness of urinary tract urothelial carcinoma increases with the severity of chronic kidney disease. BJU Int 2009;104:1471-4. [Crossref] [PubMed]
- Stefanovic V, Polenakovic M, Toncheva D. Urothelial carcinoma associated with Balkan endemic nephropathy. A worldwide disease. Pathol Biol (Paris) 2011;59:286-91. [Crossref] [PubMed]
- Chen CY, Liao YM, Tsai WM, et al. Upper urinary tract urothelial carcinoma in eastern Taiwan: high proportion among all urothelial carcinomas and correlation with chronic kidney disease. J Formos Med Assoc 2007;106:992-8. [Crossref] [PubMed]
- Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 2009;16:187-91. [Crossref] [PubMed]
- Li CC, Chang TH, Wu WJ, et al. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol 2008;54:1127-34. [Crossref] [PubMed]
- Holmang S, Johansson SL. Bilateral metachronous ureteral and renal pelvic carcinomas: incidence, clinical presentation, histopathology, treatment and outcome. J Urol 2006;175:69-72; discussion 72-3. [Crossref] [PubMed]
- Kang CH, Yu TJ, Hsieh HH, et al. The development of bladder tumors and contralateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer 2003;98:1620-6. [Crossref] [PubMed]
- Miyake H, Hara I, Kamidono S, et al. Multifocal transitional cell carcinoma of the bladder and upper urinary tract: molecular screening of clonal origin by characterizing CD44 alternative splicing patterns. J Urol 2004;172:1127-9. [Crossref] [PubMed]
- Habuchi T, Takahashi R, Yamada H, et al. Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet 1993;342:1087-8. [Crossref] [PubMed]


