Androgen deficiency and metabolic syndrome in men
Introduction
Metabolic syndrome (MetS) is a multi-system disorder marked by combinations of glucose intolerance, obesity, hypertension, and dyslipidemia. Broad medical awareness of this condition has come hand in hand with the rapidly expanding population of adults afflicted with obesity and diabetes. Initially linked to risk factors for cardiovascular disease, the components of MetS have been implicated in a range of other disease processes. Study of men with MetS has indicated a strong relationship to adult androgen deficiency and late-onset hypogonadism (LOH). This review intends to summarize our current understanding on the epidemiology, pathophysiology, and dual treatment of these disorders.
Definitions
Metabolic syndrome (MetS)
A singular definition of MetS does not exist. Medical organizations have developed diagnostic criteria with specific, and different, end-goals such as prediction of cardiovascular events or subsequent development of diabetes (1). There are four major definitions of MetS (Table 1), developed by the World Health Organization (WHO) (2), the European Group for the Study of Insulin Resistance (3), the Adult Treatment Panel of the National Cholesterol Education Program (NCEP-ATPIII) (4), and the International Diabetes Federation Consensus Group (5). Across all of these definitions are common elements, including insulin resistance, central obesity, hypertension, and dyslipidemia. For this review, we focused on studies involving men that formally meet MetS criteria, as well as men afflicted with varying degrees of individual components (i.e., obesity).

Full table
Androgen deficiency
Defining androgen deficiency is a somewhat daunting task, as the physiologic, pathologic, and biochemical implications of this definition are complex and still evolving. For the purpose of this review, androgen deficiency refers to testosterone (T) deficiency, and indicates failure of the testes to produce T due to either (I) an impairment of the hypothalamic/pituitary axis; or (II) a local deficit of the testes (6). From a clinical perspective, androgen deficiency is intrinsically linked to male hypogonadism, which is the group of signs and symptoms specifically due to either androgen deficiency or impaired action of sex steroids in effector organs (6).
Male hypogonadism consists of a spectrum of disorders that vary depending on when androgen deficiency occurs. In the context of the MetS, this specifically refers to LOH. LOH is due to low androgen levels beginning after puberty and therefore do not affect development of male anatomy or secondary sex characteristics (7). The symptoms of LOH are vague, non-specific, and commonly overlap with symptoms of aging. These symptoms include, but are not limited to, sexual symptoms, such as low libido and erectile dysfunction, loss of muscle mass and bone density, depression, and hot flashes (Table 2) (8). Due to the vague nature of these symptoms, questionnaires are generally plagued by low specificity (30-59%) (9). Nevertheless, in a population-based study of 3,369 men, the three sexual symptoms of poor morning erections, low sexual desire, and erectile dysfunction were found to have a syndromic association with low T levels (10). Assessment of symptoms is therefore considered an integral factor when deciding on whom to perform laboratory T testing; asymptomatic, healthy men generally do not qualify (11,12).

Full table
Prior to discussing specific values used to define androgen deficiency, it is important to highlight the complexities associated with T testing. T bioavailability is broken up into several parts. Specifically, T circulates in four forms: (I) tightly bound to sex-hormone binding globulin (SHBG) (~44%); (II) loosely bound to albumin (~50%); (III) loosely bound to corticosteroid binding globulin (CBG) (~4%), and unbound, or free testosterone (FT) (~2-3%) (13). The bioavailable, or active, T, consists of the free portion in addition to the CBG and albumin-bound T (13). There is no reference range for normal T that has been derived from a cohort of reproductive-age men with normal sexual function and fertility (13). T can vary over three orders of magnitude within the population. Further, T varies up to 50% in younger men with diurnal and seasonal variation (13). Common assays for T have less sensitivity in the low to low normal range (6).
As with MetS, several organizations have issued guidelines detailing the specific parameters that define androgen deficiency. These include the International Society for the Study of Aging Male (ISSAM), the International Society of Andrology (ISA), the European Associate of Urology (EAU), the European Society of Andrology (ESA), and the American Society of Andrology (ASA) (8,14,15). There are several generally agreed upon principles across guidelines (Table 3). Although there is no clear number separating low total testosterone (TT) from normal TT, 12 nmol/L or 360 ng/dL, is usually considered normal, while 8 nmol/L, or 240 ng/dL, is low. In men with a borderline value, assess FT, either by equilibrium dialysis, or by measurement of SHBG and calculation of FT level. Other methodologies of FT assessment are considered unreliable (6,13,15).
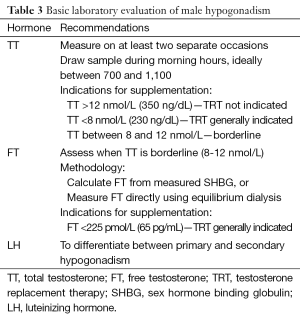
Full table
Recent investigation has focused on broader definitions of androgen deficiency/hypogonadism which consider molecular-cellular mechanisms in addition to the traditional measurement of circulating hormones (16,17). For instance, genetic variants in the androgen receptor (AR) may alter its activity, and the stability intracellular proteins, such as heat-shock protein 70 (HSP70), may subdue androgen-regulated transcription (16). Such investigations have led to a mechanistic understanding of how certain patients can exhibit symptoms of androgen deficiency in the presence of normal laboratory testing (16).
Epidemiology
According to CDC data, approximately 34% of United States adults meet NCEP/ATPIII criteria for MetS (18). The prevalence increases with age, and more than 50% of men above 60 years of age have the condition (18). Individual components of MetS are becoming increasingly common. For example, obesity rose from 27.5% of U.S. adult men in 2000 to 35.5% of U.S. adult men in 2010 (19); prevalence of MetS is considered to mirror this trend.
Epidemiologic data regarding androgen deficiency is not as robust as that for MetS. The true prevalence is unknown, with reports varying between 2.1% and 40% of adult men (6). Different definitions of androgen deficiency employed and populations studied likely account for the range in reported prevalence. The occurrence of androgen deficiency is much higher in men who have MetS than their healthy counterparts (7), and this trend has been shown reliably. Barrett-Connor, et al. reported on a group of men aged 40-79 where TT level below 350 ng/dL was found in 21% of diabetic men vs. only 13% of non-diabetic men (20). In a study of symptomatic men over 73 years of age, TT levels under 300 ng/dL were found in 64% of men with DM, but only 38% of non-diabetic men (21). The Study of Health In Pomerania (SHIP) showed that men with low TT concentrations showed a highest risk of incident MetS (22). This trend is so prominent that presence of MetS is considered an independent indication for T screening by most guidelines (8,14,15).
Mechanism and pathophysiology
The link between MetS and androgen deficiency is clear, but the exact mechanism by which men with MetS develop low T is most likely multifactorial. Several mechanisms have been elucidated (Table 4).

Full table
Leptin
MetS may influence hypogonadism through leptin, a hormone produced in adipocytes. Leptin acts on the hypothalamus, regulating energy intake (23). Leptin levels have been directly correlated to body mass index (BMI) (24). Interestingly, leptin concentration varies inversely with serum T, even when controlling for BMI and insulin levels (25). Leptin has direct, receptor-mediated actions on leydig cells in rats, providing a likely mechanism for the clinical observations in humans (26). Rat leydig cells incubated with leptin had a significant decrease in β-HCG mediated T production (26).
Inflammation
MetS is associated with a systemic inflammatory state, with increased levels of IL1, IL6, and TNF-α (27,28). Inflammatory agents predominantly affect testicular steroid production. TNF-α inhibits steroid-mediated transcription in leydig cells through NF-kB (29). IL1 inhibits cytochrome P450 mediated cholesterol side chain cleavage in leydig cells (30).
Aromatase
P450 aromatase is highly expressed in fat tissue, and catalyzes the conversion of T to estrogen. Men with MetS and central obesity develop estrogen-induced negative feedback on the hypothalamus and pituitary. Aromatase inhibitor use raised T level in obese men in several studies (31-34).
Decreased sex-hormone binding globulin
Insulin has been shown to decrease SHBG in hepatocytes in vitro (35). In a prospective analysis of men from the Massachusetts Aging Study, lower levels of both FT and SHBG independently predicted subsequent diabetes. The odds ratio (OR) for diabetes was 1.89 for a 1 SD decrease in SHBG [95% CI (range, 1.14-3.14)]. SHBG increases with age, but obese men had a slower age-adjusted increase in SHBG, leading to more rapid decline in TT with age (36).
Sleep apnea
Obstructive sleep apnea (OSA) has been correlated with MetS (37). Multiple studies have associated OSA with low baseline T levels in men (38,39). Researchers recently found apnea indicators, such as hypopnea index and percent time below SpO2 90% and 80%, to be independently associated with decreased T levels (40). Prospective data regarding the efficacy of OSA treatment on T levels is mixed. Several studies report increase of T with continuous positive pressure ventilation (CPAP) (41,42), whereas other studies note a change in SHBG, prolactin, or sexual function parameters independent of T (43-45). OSA suppresses the hypothalamic pituitary axis, disrupting LH secretion and inducing hypogonadotropic androgen deficiency (46).
Endogenous opiods (extremely obese)
In extremely obese men, the opioid antagonist naloxone was found to increase LH by 43%, indicating that endogenous opioids found in the morbidly obese may contribute to a hypogonadal state (47).
Direct effect on testicular environment
In men with MetS associated obesity, T levels may be further impaired as fat deposition in the lower abdomen increases testicular temperature (48).
Other
It should be noted that a number of studies have suggested that androgen deficiency causes MetS, rather than vice versa. SHIP for instance showed that men with low TT concentrations demonstrated the highest risk of incident MetS (22).
Treatment
Many treatments for MetS-associated androgen deficiency lack efficacy data from randomized controlled trials (RCTs). Comparing existing studies can be difficult, as definitions, patient populations, and treatment goals are heterogeneous. There are two major ways of categorizing treatments: (I) by which disease process is targeted, either MetS or androgen deficiency directly; (II) non-surgical vs. surgical interventions.
Behavioral modification
A number of RCTs have assessed the effect of weight loss on androgen levels (48-53). Results are conflicting; some studies demonstrate T rise with low-calorie diet (52,53), while others showing no change (48,49,51). A recent meta-analysis found that weight loss improved TT in obese men (P<0.0001) (54). Meta-regression analysis of the included studies found testosterone rose more in younger men and men without diabetes (P<0.0001) (54). Consistent with previously mentioned mechanisms, weight loss resulted in decreased estradiol and increased gonadotropin levels (54). Niskanen et al. showed that the improvement in testosterone levels was directly correlated with the degree of weight loss (55).
Metformin
Metformin is a commonly prescribed diabetes medication. Data regards its utility in treating androgen deficiency is conflicting. Ozata et al. administered metformin 850 mg twice daily to 40 obese men for three months in conjunction with a hypocaloric diet. They noted a decrease in FT in obese non-diabetic men and a decrease in TT in obese men with type 2 diabetes (56). Casulari et al. studied 35 men with MetS following four months of metformin 850 mg BID and a normo-caloric diet. Both FT and TT increased, regardless of whether or not they were baseline androgen deficient (57). Morgante et al. gave 45 men with MetS metformin for six months. At the end of the study period, both FT and semen parameters were significantly improved (58). TT rose an average of 0.9 ng/mL (P<0.02), FT rose an average of 14 pg/mL (P<0.001), and semen concentration, % motility, and normal morphology all increased (P<0.001) (58).
Gonadotropin and Gonadotropin-releasing hormone (GnRH)
Gonadotropin and pulsatile GnRH administration has been used in men with LOH who desire fertility. Due to cost and complexity of administration, these agents are no considered first line therapy in absence of fertility concerns. Data regarding the use of these agents in men with MetS are limited (59).
Testosterone replacement therapy (TRT)
To date, there are six RCTs assessing the effect of TRT on men with MetS or diabetes (52,60-64). Combined, these RCTs include 483 patients with an average of 57 weeks follow-up. A recent meta-analysis of this data showed that TRT improved fasting blood glucose by a mean of 0.48 mmol/L (P<0.001), lowered triglyceride levels by a mean of 0.4 nmol/L (P<0.001), and reduced waist circumference by approximately 4.1 cm (P=0.03) in men with MetS (6). From a pathophysiologic standpoint, TRT has been shown to decrease cytokine production in men with MetS; TRT may positively impact these individuals through an anti-inflammatory effect (7).
Antiestrogens
In the male, selective estrogen receptor modulators (SERMs) exert an antagonist effect on estrogen receptors in the hypothalamus and pituitary gland that regulate gonadotropin release. SERM administration leads to increased FSH, LH and testis activity (6). Guay et al. demonstrated that treatment with clomiphene citrate increased LH, FSH, TT and FT significantly in men with secondary hypogonadism and ED (65). Given the estrogen-mediated component of hypogonadism in MetS, this may represent a useful therapy in this population.
Surgery: bariatric
The positive impact of bariatric surgery on TT levels appear to be greater than those of non-surgical weight loss and are associated with significant improvement of erectile function and sex-related quality of life (49,66). A recent meta-analysis, for example, demonstrated improved TT following bariatric surgery, with a more significant association between TT rise and bariatric surgery than between TT rise and low-calorie diet (54). Only two RCTs exist analyzing the connection between bariatric surgery and hypogonadism (49,50). Reis et al. randomized 20 men to either gastric bypass or control, and noted increased TT, FT, and FSH in men undergoing surgery relative to control at 20 months follow-up (49). Mingrone et al. randomized 27 men to either diet or malabsorptive surgery and followed them for one year. Men undergoing malabsorptive surgery had a significant increase in SHBG level, with an average rise of 40 nmol/L (P<0.0001), whereas men randomized to low-calorie diet did not (50).
Surgery: varicocelectomy
Varicocele has traditionally been associated with male infertility, although more recent data suggests that it may be a risk factor for low T levels. The exact pathophysiology of the negative effects of varicocele on testicular function, however, is not well understood, and the empiric experience has been limited to infertile patients. Recent data suggests that microsurgical varicocelectomy improves T levels in infertile men with varicocele (64,67,68).
Ozturk et al. investigated the influence of MetS on success of varicocele repair. A total of 56 men without MetS vs. 48 men with MetS underwent varicocele repair. Spontaneous pregnancy rate at two years follow up was 45% in the non-MetS group vs. 34% in the MetS group (P<0.05) (69). Varicocelectomy may improve T levels in fertile patients with hypogonadism, but empiric evidence is currently lacking.
Summary
MetS is a growing health concern worldwide. Initially a point of interest in cardiovascular events, the cluster of HTN, obesity, dyslipidemia, and insulin resistance known as MetS has become associated with a variety of other disease processes, including androgen deficiency and LOH. Men with MetS are at a higher risk of developing androgen deficiency, and routine screening of T is advised in this population. The pathophysiology of androgen deficiency in MetS is multifactorial, and consists of inflammatory, enzymatic, and endocrine derangements. Many options for the concomitant treatment of both disorders exist. Direct treatment of androgen deficiency with MetS, whether by diet, exercise or surgery, will improve T levels. Conversely, TRT has been shown to treat MetS in multiple RTCs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Corona G, Mannucci E, Forti G, et al. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl 2009;32:587-98. [PubMed]
- Consultation W. Definition, diagnosis and classification of diabetes mellitus and its complications. Geneva, Switzerland: World Health Organization 1999;31:1-59.
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999;16:442-3. [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421. [PubMed]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469-80. [PubMed]
- Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab 2013;27:557-79. [PubMed]
- Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes 2007;14:226-34. [PubMed]
- Dohle GR, Arver S, Bettocchi C, et al. Guidelines on male hypogonadism. Euro Ass Urol 2012. Accessed online: http://www.uroweb.org/gls/pdf/16_Male_Hypogonadism_LR%20II.pdf
- Morley JE, Perry HM 3rd, Kevorkian RT, et al. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424-9. [PubMed]
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35. [PubMed]
- Buvat J, Maggi M, Guay A, et al. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med 2013;10:245-84. [PubMed]
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335-43. [PubMed]
- Paduch DA, Brannigan RE, Fuchs EF, et al. The Laboratory Diagnosis of Testosterone Deficiency. Urology 2014. [Epub ahead of print]. [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [PubMed]
- Wang C, Nieschlag E, Swerdloff R, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Impot Res 2009;21:1-8. [PubMed]
- Carruthers M. Testosterone deficiency syndrome: cellular and molecular mechanism of action. Curr Aging Sci 2013;6:115-24. [PubMed]
- Rey RA, Grinspon RP, Gottlieb S, et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology 2013;1:3-16. [PubMed]
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report 2009.1-7. [PubMed]
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief 2012.1-8. [PubMed]
- Barrett-Connor E, Khaw KT, Yen SS. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol 1990;132:895-901. [PubMed]
- Tan RS, Pu SJ. Impact of obesity on hypogonadism in the andropause. Int J Androl 2002;25:195-201. [PubMed]
- Haring R, Ernst F, Schurmann C, et al. The androgen receptor CAG repeat polymorphism as a risk factor of low serum testosterone and its cardiometabolic effects in men. Int J Androl 2012;35:511-20. [PubMed]
- Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab 2009;296:E1230-8. [PubMed]
- McConway MG, Johnson D, Kelly A, et al. Differences in circulating concentrations of total, free and bound leptin relate to gender and body composition in adult humans. Ann Clin Biochem 2000;37:717-23. [PubMed]
- Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab 1998;83:3243-6. [PubMed]
- Caprio M, Isidori AM, Carta AR, et al. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology 1999;140:4939-47. [PubMed]
- Alexandraki K, Piperi C, Kalofoutis C, et al. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci 2006;1084:89-117. [PubMed]
- Giulietti A, Stoffels K, Decallonne B, et al. Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann N Y Acad Sci 2004;1037:74-8. [PubMed]
- Hong CY, Park JH, Ahn RS, et al. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 2004;24:2593-604. [PubMed]
- Lin T, Wang D, Stocco DM. Interleukin-1 inhibits Leydig cell steroidogenesis without affecting steroidogenic acute regulatory protein messenger ribonucleic acid or protein levels. J Endocrinol 1998;156:461-7. [PubMed]
- de Boer H, Verschoor L, Ruinemans-Koerts J, et al. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab 2005;7:211-5. [PubMed]
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 2003;52:1126-8. [PubMed]
- Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 2008;158:741-7. [PubMed]
- Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Clin Pract Endocrinol Metab 2008;4:415-9. [PubMed]
- Plymate SR, Matej LA, Jones RE, et al. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 1988;67:460-4. [PubMed]
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 2000;23:490-4. [PubMed]
- Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev 2005;9:211-24. [PubMed]
- Luboshitzky R, Lavie L, Shen-Orr Z, et al. Altered luteinizing hormone and testosterone secretion in middle-aged obese men with obstructive sleep apnea. Obes Res 2005;13:780-6. [PubMed]
- Luboshitzky R, Zabari Z, Shen-Orr Z, et al. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab 2001;86:1134-9. [PubMed]
- Hammoud AO, Walker JM, Gibson M, et al. Sleep apnea, reproductive hormones and quality of sexual life in severely obese men. Obesity (Silver Spring) 2011;19:1118-23. [PubMed]
- Grunstein RR, Handelsman DJ, Lawrence SJ, et al. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab 1989;68:352-8. [PubMed]
- Luboshitzky R, Lavie L, Shen-Orr Z, et al. Pituitary-gonadal function in men with obstructive sleep apnea. The effect of continuous positive airways pressure treatment. Neuro Endocrinol Lett 2003;24:463-7. [PubMed]
- Hoekema A, Stel AL, Stegenga B, et al. Sexual function and obstructive sleep apnea-hypopnea: a randomized clinical trial evaluating the effects of oral-appliance and continuous positive airway pressure therapy. J Sex Med 2007;4:1153-62. [PubMed]
- Macrea MM, Martin TJ, Zagrean L. Infertility and obstructive sleep apnea: the effect of continuous positive airway pressure therapy on serum prolactin levels. Sleep Breath 2010;14:253-7. [PubMed]
- Meston N, Davies RJ, Mullins R, et al. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med 2003;254:447-54. [PubMed]
- Luboshitzky R, Aviv A, Hefetz A, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab 2002;87:3394-8. [PubMed]
- Blank DM, Clark RV, Heymsfield SB, et al. Endogenous opioids and hypogonadism in human obesity. Brain Res Bull 1994;34:571-4. [PubMed]
- Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc 1999;31:1320-9. [PubMed]
- Reis LO, Favaro WJ, Barreiro GC, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl 2010;33:736-44. [PubMed]
- Mingrone G, Greco AV, Giancaterini A, et al. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis 2002;161:455-62. [PubMed]
- Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 2011;8:2868-75. [PubMed]
- Heufelder AE, Saad F, Bunck MC, et al. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726-33. [PubMed]
- Kaukua J, Pekkarinen T, Sane T, et al. Sex hormones and sexual function in obese men losing weight. Obes Res 2003;11:689-94. [PubMed]
- Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 2013;168:829-43. [PubMed]
- Niskanen L, Laaksonen DE, Punnonen K, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004;6:208-15. [PubMed]
- Ozata M, Oktenli C, Bingol N, et al. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes Res 2001;9:662-7. [PubMed]
- Casulari LA, Caldas AD, Domingues Casulari Motta L, et al. Effects of metformin and short-term lifestyle modification on the improvement of male hypogonadism associated with metabolic syndrome. Minerva Endocrinol 2010;35:145-51. [PubMed]
- Morgante G, Tosti C, Orvieto R, et al. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil Steril 2011;95:2150-2. [PubMed]
- Hong BS, Ahn TY. Recent trends in the treatment of testosterone deficiency syndrome. Int J Urol 2007;14:981-5. [PubMed]
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828-37. [PubMed]
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602-12. [PubMed]
- La Vignera S, Calogero AE, D’Agata R, et al. Testosterone therapy improves the clinical response to conventional treatment for male patients with metabolic syndrome associated to late onset hypogonadism. Minerva Endocrinol 2008;33:159-67. [PubMed]
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7:3495-503. [PubMed]
- Aversa A, Bruzziches R, Francomano D, et al. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest 2010;33:776-83. [PubMed]
- Guay AT, Jacobson J, Perez JB, et al. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res 2003;15:156-65. [PubMed]
- Rao SR, Kini S, Tamler R. Sex hormones and bariatric surgery in men. Gend Med 2011;8:300-11. [PubMed]
- Hsiao W, Rosoff JS, Pale JR, et al. Varicocelectomy is associated with increases in serum testosterone independent of clinical grade. Urology 2013;81:1213-7. [PubMed]
- Goldstein M, Tanrikut C. Microsurgical management of male infertility. Nat Clin Pract Urol 2006;3:381-91. [PubMed]
- Ozturk U, Sener NC, Nalbant I, et al. The effect of metabolic syndrome upon the success of varicocelectomy. ScientificWorldJournal 2012;2012:985201.


