Innovative approaches for complex penile urethral strictures
Introduction: etiology and demographics
Patients with strictures have been shown to account for a sizable burden on the healthcare system with 1.5 million office visits over an 8-year period in addition to 5,000 inpatient visits annually. The economic impact is significant as well, with an estimated annual cost of around $191 million in the year 2000 (1).
Strictures of the anterior urethra are most common, accounting for 92% of cases. Within the anterior urethra, bulbar strictures occur most often (46.9%), followed by penile (30.5%) and combined bulbar/penile (9.9%) (2). The etiology of urethral strictures is highly variable and largely depends on stricture location. Penile urethral strictures are most commonly caused by inflammatory conditions (40%), such as lichen sclerosis, and iatrogenic injury (40%). In contrast, an idiopathic etiology is most commonly observed in cases of bulbar disease (40%), followed by iatrogenic (35%) and inflammatory causes (10%) (2). Urethral trauma accounts for 5% of all penile strictures and 15% of bulbar strictures in the industrialized world. However, trauma accounts for a much higher percentage of overall strictures in the developing world, secondary to blunt pelvic trauma and gunshot wounds (3). In these cases, posterior urethral injury is often observed.
Management approach to anterior urethral strictures
Initial management of anterior urethral strictures often involves trials of minimally invasive therapies such as dilation and internal urethrotomy. Dilation techniques may involve catheters, filiforms with followers, balloon dilators, and/or urethral sounds. Direct vision internal urethrotomy (DVIU) involves incising the narrowed urethral segment endoscopically and then allowing the urethral segment to heal at a larger diameter. Despite similarly poor long-term success rates with these options (0-30% for DVIU) (4-12), they continue to be the most common treatment applied to strictures of the anterior male urethra (12-15).
Multiple studies have demonstrated declining efficacy after repeated DVIU attempts, with success rates as low as 0% at four years reported after a second procedure (4-6,8,10). It also appears that multiple internal urethrotomy procedures promote increased scar formation and the possibility of a longer, more dense stricture at the time of open repair. This adverse effect, though, does not appear to effect success rates for subsequent open repair (16). Some reports have suggested that a single initial attempt with DVIU in the appropriate stricture is a cost effective approach prior to attempted open urethroplasty. Others have argued for urethroplasty as an initial management strategy in situations DVIU is likely to fail, such as cases of long strictures (>2 cm) or those located in the penile urethra (5,17-21).
Open urethral reconstruction has a high rate of success in treating strictures, with long-term patency achieved in 85-90% (22-33). As such, multiple urethroplasty techniques may be employed based on the characteristics of the strictured segment. With a high success rate (90-95%), excision and primary anastomosis (EPA) involves transection of the urethra with removal of the diseased urethra segment and reanastomosis of the spatulated urethral segments. Unfortunately EPA is limited to short bulbar strictures of 1-2 cm, where the excision will not result in penile shortening or chordee. Augmented anastomotic urethroplasty represent a viable option in cases where the stricture defect is 2 to 4 cm long. Longer strictures often require tissue substitution with grafts or flaps (3).
Complex anterior urethral strictures, including those resulting from failed hypospadias repair, prior urethroplasty, or those with obliterative urethral segments, provide a unique challenge to reconstructive urologists. These difficult cases often require complete excision of long urethral segments as well as circumferential tissue substitution. Tubularized flaps and grafts have been attempted in the past but were abandoned due to high recurrence rates approaching 50% (34,35). Given the poor results observed in initial small series, these cases have typically been managed with improved success using a 2-stage Johanson technique (36-38). This technique requires a 6-month interval between the first stage grafting and the subsequent completion stage where the neourethra is tubularized. This time interval with a severely hypospadic urethra is often undesirable to many patients. Furthermore, recent reports have shown that a large number of these patients undergoing “two-stage repair” will actually require far more procedures than just the name implies (37,38). The need for multiple procedures in patients undergoing two-stage repair as well as patient unwillingness has led to the development of some innovative approaches for challenging, long strictures of the anterior urethra.
Dorsal graft with ventral penile skin flap
The combination of dorsal buccal graft with ventral penile skin flap has been suggested for patients with long anterior urethral strictures including a severely narrowed or obliterated urethral plate. Morey initially described a one-stage approach consisting of urethral plate salvage using a dorsal buccal graft combined with a ventral penile skin flap in patients with severe pendulous urethral strictures (39). In the early utilization of this novel technique (mean follow-up 2.1 years), all four patients meeting study inclusion criteria voided without difficulty and required no additional instrumentation.
Likewise, Erickson and colleagues described a one-stage repair of anterior urethral strictures in 14 men using a combined dorsal onlay buccal graft with a ventral fasciocutaneous flap (40). The average stricture length in this group was 9.75 cm with 12 (12/14, 85%) structures located in the penile/bulbar location. At a median follow up of 2.5 years, the study investigators reported an overall success rate of 78% (11/14 patients), although two of these patients (14%) required an additional endoscopic procedure to achieve urethral patency. Patients with longer strictures appeared to be at higher risk of stricture recurrence (12.8 vs. 8.7 cm).
Gelman and associates described a similar experience treating distal obliterative strictures with a combined dorsal buccal graft and a ventral penile skin flap (n=12) (41). Though the authors did not mention stricture length, various size buccal grafts were utilized (range, 2-6.5 cm) with all strictures located in the pendulous urethra. All patients (n=12) were noted to have urethral patency on follow-up cystoscopy at three months postoperatively with normal voiding demonstrated at a mean 39 months follow-up.
Djordjevic and colleagues have also applied this technique in a pediatric hypospadias population (42). A group of 17 patients, all less than 24 months old, underwent a one-stage repair for severe hypospadias (13 penoscrotal and 4 scrotal). The similar technique involved a dorsal buccal graft combined with a ventrally applied dorsal island penile skin flap. At a mean follow up of 25 months, 14 patients (82%) achieved success while complications of urethral fistula and distal urethral stricture were observed in the remaining three patients (18%).
The overall success of a combined dorsal buccal graft with ventral penile skin flap is likely due to the optimized blood supply utilized by both components of the repair. Previous failures using tubularized grafts or flaps were thought to be due to insufficient blood supply at the edges of the graft (40). In the case of this combined technique, however, both graft and flap components have an independent, reliable blood supply. The dorsal buccal graft has been shown previously to have excellent success rates (27-33). This is largely due to its robust and evenly distributed microvascular structure which promotes inosculation and imbibition when fixed to the tunical recipient bed (27). At the same time, a fasciocutaneous flap relies on its own established blood supply originating from Buck’s fascia that is preserved during its harvest.
Nevertheless, there are limitations in the use of penile skin flaps for urethral reconstruction. Manipulation of penile skin for urethral reconstruction must be avoided in patients with lichen sclerosis and is often discouraged in patients with hypospadias. As a significant number of patients with long anterior urethral strictures have a history of such conditions, these interesting techniques often cannot be performed and utilization of buccal mucosa grafting only is paramount.
Combined dorsal and ventral buccal mucosa grafting
An alternative approach in cases of long strictures with inadequate urethral plate is a combined dorsal and ventral buccal graft. Palminteri and colleagues initially described their technique in a group of 48 patients with bulbar strictures (43). Their technique combined the dorsal inlay approach of Asopa (44) with a ventral onlay graft as described by Elliott (45). Of the initial 48 cases, 43/48 (89.6%) were successful at 22 months follow up (‘success’ defined by voiding normally without the need for any additional post-operative procedures). Onsubsequent follow up of 48.9 months, 64 of 73 patients (88%) were voiding normally. Erectile function was preserved in all patients undergoing repair (46).
Goel and colleagues, who had previously reported utilizing combined grafts for meatoplasty (47), recently compared a dorsal buccal graft placement to a combined dorsal and ventral graft placement in 20 patients with pendulous urethral strictures (48). With ten patients in each group, group 1 underwent Asopa inlay only (44) while group 2 underwent an Asopa inlay with an additional ventrally placed graft. Both groups were well matched based on stricture length (7.2 vs. 7.5 cm), etiology, and location. Success rates were comparable (7/10 vs. 8/10) at follow up of 35.7 months and 31.8 months, for groups 1 and 2 respectively. It is noteworthy that longer surgical times were noted in the combined graft group but the complication rates remained similar.
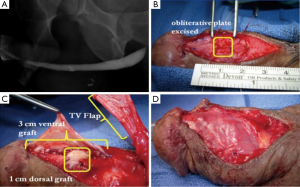
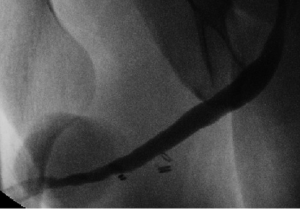
We have previously published our institution’s experience with combined dorsal and ventral buccal mucosa grafting for complex anterior urethral stricture with obliterative or near-obliterative segments (49). Mean stricture length was 4.5 cm with a varied stricture location, 39% of patients having involvement of the pendulous urethra. Our technique involved a ventral approach to the strictured urethral segment as previously described (44). In obliterative urethral segments (<5 mm width), the urethra was excised and a dorsal graft was quilted onto the corporal bodies to recreate the urethral plate (Figure 1A,B). Alternatively if the strictured segment was wider (5-10 mm), the urethral plate was divided longitudinally and a graft was quilted dorsally to enhance its width. We then completed our circumferential repair with a ventral onlay graft (Figure 1C) (24), with dartos/tunica vaginalis flap coverage in areas of insufficient spongiosum (Figure 1D). Postoperative VCUG demonstrated excellent patency of the repair (Figure 2). Of our 36 cases performed, 32 patients (89%) demonstrated successful outcomes, as defined by voiding normally without the need for additional procedures at a follow up of 15.7 months.
Overall these studies have demonstrated success in a heterogeneous population of anterior urethral strictures with respect to etiology, location and stricture length. Since penile skin is not being utilized, this technique is suitable for lichen sclerosis or hypospadias where healthy penile skin may be deficient or diseased. As previously discussed, the microvascular structure of the buccal graft leads to excellent graft take, especially when applied dorsally with the blood supply provided from the tunica. In contrast, the ventrally placed graft must rely on the spongiosum for its blood supply. In cases of deficient spongiosum, such as with hypospadias or distal strictures where the corpus spongiosum tends to be less robust, we cover the graft with a tunica vaginalis or dartos flap.
Alternative graft material
Graft material in adequate supply can be a limiting factor in long complex urethral strictures. This is especially true with lichen sclerosis or hypospadias failure where usable penile skin is scarce. When buccal mucosa has been previously harvested or is not of sufficient supply, lingual graft tissue can be utilized, with excellent results (50). Oral mucosa can also be obtained from labial grafts, with good outcomes demonstrated in pediatric patients, generally in repair of hypospadias defects (51). Similar outcomes have been shown in adults as well, with no significant difference in postoperative quality of life due to graft site complications (52).
Additionally, buccal mucosa and lingual grafts can be hard to procure in a patient with prior graft harvesting or in cases where the patient has concomitant oral disease. Regenerative medicine offers the possibility of production of patient-specific grafts, negating the necessity of graft harvesting (53).
Tissue-engineered grafts are generally divided into cellular and acellular subtypes. Acellular grafts are usually from cadaveric or animal sources and treated to make the matrix cell-free. Alternatively, cellular grafts are made by culturing a particular cell type, generally obtained via biopsy, and then populating biologic scaffolding to produce a histologically similar construct of the native tissue.
Acellular grafts have been used in the treatment of urethral stricture disease with varying success. Palminteri reported his use of porcine small intestinal mucosa as a graft material in bulbar urethroplasties of 25 men (54). At 71 months follow-up, 24% of patients failed as they required additional interventions. In particular, those with strictures >4 cm experienced a higher failure rate.
With regard to cellular tissue-engineered buccal mucosal grafts, Bhargava and colleagues attempted urethroplasty in five patients using a graft derived from an acellular human dermal matrix seeded with human oral fibroblasts and keratinocytes (55). Initial graft take was observed in all patients, however, at 33.6 months follow up, two patients required partial or full removal of the graft due to fibrosis and hyperproliferation. The other three patients had patent urethras but only after additional instrumentation to correct strictures that developed from graft contraction. It is noteworthy that all patients in this study had strictures caused by lichen sclerosis, potentially causing poorer outcomes.
Various techniques have also been employed in the production of tissue-engineered urothelial grafts for the treatment of urethral stricture disease (56,57). In particular, Raya-Rivera and colleagues were able to create autologous urethras on a biologic scaffold, populated with bladder epithelial and muscle cells taken from bladder biopsies (58). These neourethras were then used in the repair of five boys with traumatic posterior urethral injuries. Biopsies of the reconstructed urethras showed similar histologic characteristics to native urethras. Additionally cystoscopy at 72 months demonstrated patency in all five boys. While these results are encouraging, there are clear limitations. Further investigation of these tissue-engineered grafts needs to take place with defined stricture characteristics (etiology, size, location), larger patient numbers, and direct comparison to traditional graft tissues.
Summary
Complex anterior urethra strictures with obliterative segments continue to be a challenging clinical scenario for reconstructive urologists. Past approaches such as tubularized flaps and grafts had an unacceptably high failure rate. Two-stage approaches, while a reasonable option, are not uniformly acceptable to many patients due to the interval between the initial and definitive procedures and treatment fatigue caused by multiple operations. Combined dorsal and ventral buccal grafting or ventral penile flaps have proven successful in these difficult cases and represent viable one-stage options. Further long-term follow up and comparative trials are necessary to fully evaluate these promising techniques. Finally, in the future, the reconstructive urologist is likely to employ more engineered graft material, tailored to each individual patient. Investigation is pending in these innovative materials; however, they do offer the potential of freeing the surgeon from the limitations of current graft options, providing an abundant, non-harvested supply for long, complex repairs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667-74. [PubMed]
- Palminteri E, Berdondini E, Verze P, et al. Contemporary urethral stricture characteristics in the developed world. Urology 2013;81:191-6. [PubMed]
- Barbagli G, Sansalone S, Djinovic R, et al. Current controversies in reconstructive surgery of the anterior urethra: a clinical overview. Int Braz J Urol 2012;38:307-16. [PubMed]
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol 1996;156:73-5. [PubMed]
- Albers P, Fichtner J, Brühl P, et al. Long-term results of internal urethrotomy. J Urol 1996;156:1611-4. [PubMed]
- Heyns CF, Steenkamp JW, De Kock ML, et al. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol 1998;160:356-8. [PubMed]
- Tonkin JB, Jordan GH. Management of distal anterior urethral strictures. Nat Rev Urol 2009;6:533-8. [PubMed]
- Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol 2010;183:1859-62. [PubMed]
- Dubey D. The current role of direct vision internal urethrotomy and self-catheterization for anterior urethral strictures. Indian J Urol 2011;27:392-6. [PubMed]
- Veeratterapillay R, Pickard RS. Long-term effect of urethral dilatation and internal urethrotomy for urethral strictures. Curr Opin Urol 2012;22:467-73. [PubMed]
- Wong SS, Aboumarzouk OM, Narahari R, et al. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database Syst Rev 2012;12:CD006934. [PubMed]
- Hampson LA, McAninch JW, Breyer BN. Male urethral strictures and their management. Nat Rev Urol 2014;11:43-50. [PubMed]
- Anger JT, Buckley JC, Santucci RA, et al. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology 2011;77:481-5. [PubMed]
- Anger JT, Scott VC, Sevilla C, et al. Patterns of management of urethral stricture disease in the Veterans Affairs system. Urology 2011;78:454-8. [PubMed]
- Naudé AM, Heyns CF. What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat Clin Pract Urol 2005;2:538-45. [PubMed]
- Barbagli G, Palminteri E, Lazzeri M, et al. Long-term outcome of urethroplasty after failed urethrotomy versus primary repair. J Urol 2001;165:1918-9. [PubMed]
- Wright JL, Wessells H, Nathens AB, et al. What is the most cost-effective treatment for 1 to 2-cm bulbar urethral strictures: societal approach using decision analysis. Urology 2006;67:889-93. [PubMed]
- Wessells H. Cost-effective approach to short bulbar urethral strictures supports single internal urethrotomy before urethroplasty. J Urol 2009;181:954-5. [PubMed]
- Greenwell TJ, Castle C, Andrich DE, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol 2004;172:275-7. [PubMed]
- Rourke KF, Jordan GH. Primary urethral reconstruction: the cost minimized approach to the bulbous urethral stricture. J Urol 2005;173:1206-10. [PubMed]
- Morey A. Urethral stricture is now an open surgical disease. J Urol 2009;181:953-4. [PubMed]
- Wood DN, Andrich DE, Greenwell TJ, et al. Standing the test of time: the long-term results of urethroplasty. World J Urol 2006;24:250-4. [PubMed]
- Andrich DE, Mundy AR. What is the best technique for urethroplasty? Eur Urol 2008;54:1031-41. [PubMed]
- Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: analysis of 168 patients. J Urol 2002;167:1715-9. [PubMed]
- Morey AF, Kizer WS. Proximal bulbar urethroplasty via extended anastomotic approach--what are the limits? J Urol 2006;175:2145-9. [PubMed]
- Eltahawy EA, Virasoro R, Schlossberg SM. J Urol 2007;177:1803-6. [PubMed]
- Barbagli G, Palminteri E, Rizzo M. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa in adult bulbourethral strictures. J Urol 1998;160:1307-9. [PubMed]
- Markiewicz MR, Lukose MA, Margarone JE 3rd, et al. The oral mucosa graft: a systematic review. J Urol 2007;178:387-94. [PubMed]
- Barbagli G, Palminteri E, Guazzoni G, et al. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol 2005;174:955-7. [PubMed]
- Barbagli G, Morgia G, Lazzeri M. Retrospective outcome analysis of one-stage penile urethroplasty using a flap or graft in a homogeneous series of patients. BJU Int 2008;102:853-60. [PubMed]
- Zimmerman WB, Santucci RA. Buccal mucosa urethroplasty for adult urethral strictures. Indian J Urol 2011;27:364-70. [PubMed]
- Mangera A, Patterson JM, Chapple CR. A systematic review of graft augmentation urethroplasty techniques for the treatment of anterior urethral strictures. Eur Urol 2011;59:797-814. [PubMed]
- Lumen N, Oosterlinck W, Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int 2012;89:387-94. [PubMed]
- Wiener JS, Sutherland RW, Roth DR, et al. Comparison of onlay and tubularized island flaps of inner preputial skin for the repair of proximal hypospadias. J Urol 1997;158:1172-4. [PubMed]
- Carney KJ, McAninch JW. Penile circular fasciocutaneous flaps to reconstruct complex anterior urethral strictures. Urol Clin North Am 2002;29:397-409. [PubMed]
- Jaffar DJ, Sewell GR, Schwarz FW. Johanson urethroplasty for repair of urethral strictures. J Urol 1956;75:805-10. [PubMed]
- Kozinn SI, Harty NJ, Zinman L, et al. Management of complex anterior urethral strictures with multistage buccal mucosa graft reconstruction. Urology 2013;82:718-22. [PubMed]
- Andrich DE, Greenwell TJ, Mundy AR. The problems of penile urethroplasty with particular reference to 2-stage reconstructions. J Urol 2003;170:87-9. [PubMed]
- Morey AF. Urethral plate salvage with dorsal graft promotes successful penile flap onlay reconstruction of severe pendulous strictures. J Urol 2001;166:1376-8. [PubMed]
- Erickson BA, Breyer BN, McAninch JW. Single-stage segmental urethral replacement using combined ventral onlay fasciocutaneous flap with dorsal onlay buccal grafting for long segment strictures. BJU Int 2012;109:1392-6. [PubMed]
- Gelman J, Sohn W. 1-stage repair of obliterative distal urethral strictures with buccal graft urethral plate reconstruction and simultaneous onlay penile skin flap. J Urol 2011;186:935-8. [PubMed]
- Djordjevic ML, Majstorovic M, Stanojevic D, et al. Combined buccal mucosa graft and dorsal penile skin flap for repair of severe hypospadias. Urology 2008;71:821-5. [PubMed]
- Palminteri E, Manzoni G, Berdondini E, et al. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol 2008;53:81-9. [PubMed]
- Asopa HS, Garg M, Singhal GG, et al. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology 2001;58:657-9. [PubMed]
- Elliott SP, Metro MJ, McAninch JW. Long-term followup of the ventrally placed buccal mucosa onlay graft in bulbar urethral reconstruction. J Urol 2003;169:1754-7. [PubMed]
- Palminteri E, Berdondini E, Shokeir AA, et al. Two-sided bulbar urethroplasty using dorsal plus ventral oral graft: urinary and sexual outcomes of a new technique. J Urol 2011;185:1766-71. [PubMed]
- Goel A, Dalela D, Sankhwar SN. Meatoplasty using double buccal mucosal graft technique. Int Urol Nephrol 2009;41:885-7. [PubMed]
- Goel A, Jain A. Buccal mucosal graft urethroplasty for penile stricture: only dorsal or combined dorsal and ventral graft placement? Urology 2011;77:1482-6. [PubMed]
- Hudak SJ, Lubahn JD, Kulkarni S, et al. Single-stage reconstruction of complex anterior urethral strictures using overlapping dorsal and ventral buccal mucosal grafts. BJU Int 2012;110:592-6. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. The use of lingual mucosal graft in adult anterior urethroplasty: surgical steps and short-term outcome. Eur Urol 2008;54:671-6. [PubMed]
- Snodgrass WT, Bush N, Cost N. Algorithm for comprehensive approach to hypospadias reoperation using 3 techniques. J Urol 2009;182:2885-91. [PubMed]
- Jang TL, Erickson B, Medendorp A, et al. Comparison of donor site intraoral morbidity after mucosal graft harvesting for urethral reconstruction. Urology 2005;66:716-20. [PubMed]
- Mangera A, Chapple CR. Tissue engineering in urethral reconstruction--an update. Asian J Androl 2013;15:89-92. [PubMed]
- Palminteri E, Berdondini E, Fusco F, et al. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 2012;79:695-701. [PubMed]
- Bhargava S, Patterson JM, Inman RD, et al. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol 2008;53:1263-9. [PubMed]
- Nagele U, Maurer S, Feil G, et al. In vitro investigations of tissue-engineered multilayered urothelium established from bladder washings. Eur Urol 2008;54:1414-22. [PubMed]
- Li C, Xu YM, Liu ZS, et al. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 2013;81:1075-80. [PubMed]
- Raya-Rivera A, Esquiliano DR, Yoo JJ, et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 2011;377:1175-82. [PubMed]


