
Penile vascular abnormalities in young men with persistent side effects after finasteride use for the treatment of androgenic alopecia
Introduction
5ARIs are approved for treatment of benign prostatic hyperplasia (BPH) and AGA. 5ARIs inhibit the conversion of testosterone to dihydrotestosterone (DHT) through competitive inhibition of the enzyme 5α-reductase (1,2). Although 5ARIs can be highly beneficial in the above populations, they are associated with known adverse effects including decreased libido and erectile dysfunction (ED). Given concerns regarding persistent adverse sexual side effects, the FDA amended the 5ARI package insert label to include a warning of sexual dysfunction that may persist after stopping the medication (2). This is not a negligible risk, which has led to the creation of patient advocacy groups that intend to understand, explain, and possibly help treat persistent 5ARI side effects (3).
PFS describes the persistent negative sexual, neurological, and physical symptoms in patients who have taken 5ARIs (3). Although sexual side effects of 5ARIs are well described in the literature, many questions still exist surrounding other side effects including severe depression (4), cognitive decline and genitourinary and musculoskeletal complaints. A recent review indicated that finasteride may impact the physical, psychiatric, and cognitive domains (5) in addition to sexual and genital function.
Most of the current literature on PFS supports increased rates of ED and low libido in patients taking 5ARIs (6). More worrisome, however, are data observing an increased risk of persistent sexual side effects despite discontinuation of the medication (7,8). In addition to ED and low libido, adverse effects of finasteride may include penile atrophy, diminished ejaculatory volume and force, and an increased incidence of Peyronie’s disease; the latter of which may be mediated by a decreased effect of DHT on male genitalia (9-11).
The effects of finasteride on BPH are well established in the literature (12). Finasteride acts at the level of 5α-reductase, blocking the conversion of testosterone to DHT. There are two main 5α-reductase isotypes with localized effects. Broadly speaking, type 1 5α-reductase affects most tissues of the body while type 2 specifically affects genital tissue, namely the prostate (13). As a selective type 2 5ARI, finasteride acts primarily on genital tissue, reducing the level of DHT primarily in the prostate, leading to a low androgen state within the prostate.
Patients who have taken finasteride also report changes in body composition. Male gynecomastia is commonly reported (9,11). Physical changes appear to stem from aromatization of testosterone that is not metabolized by the 5α-reductase pathway (14). Additionally, patients commonly report muscle weakness and twitching, as well as increased fat deposition after stopping finasteride—though objective assessments have not yielded measurable changes in fat distribution or strength (9,15).
Psychiatric and cognitive changes reported by PFS patients may be mediated by 5ARI effects on neurosteroid and neurotransmitter levels (15). These neurohormonal changes could contribute to the high rates of depressive symptoms reported by men after 5ARI use (15,16). In addition, 5ARI use has been correlated with higher rates of self-reported anxiety as well as memory and attention disturbances (9,11,15). Of note, no significant decrease in cognitive performance was appreciated after evaluation of these patients (15). A recent study found high rates of a previous psychiatric diagnosis in PFS patients or in first-degree relatives, suggesting a possible risk factor for developing PFS (14).
Given the wide range of symptoms after the initiation of finasteride, the present study sought to further describe the persistent sexual, genitourinary, physical, psycho-cognitive, and anti-androgenic effects of 5ARIs in men treated for AGA.
Methods
We performed a prospective case-control study to evaluate the effects of 5ARIs on multiple men’s health parameters. Twenty-five subjects who took 5ARIs for AGA (experimental group) were compared to 28 controls who did not take 5ARIs (control group). All 25 subjects in the experimental group took finasteride and one took dutasteride after stopping finasteride. Twenty-five control patients who had not taken 5ARIs and were separately being evaluated for circumcision were included.
The study was approved by the Institutional Review Board of Baylor College of Medicine and informed consent was taken from all the patients prior to the start of the study. The study took place at the Baylor College of Medicine Medical Center Urology Clinic in Houston, Texas from March 2013 to September 2018. Eligibility criteria included men over 18 years of age who were being seen for sexual dysfunction at the primary investigator’s (M Khera) clinic.
Sexual, genitourinary, physical, psychiatric, cognitive, and androgenic characteristics were analyzed using validated questionnaires or self-reported complaints. Sexual function was assessed using patient-reported sexual complaints, the validated International Index of Erectile Function (IIEF) questionnaire scores and penile duplex Doppler ultrasound (PDDU). PDDU was performed by administering intracavernosal injections to induce erection and recording peak systolic and end diastolic velocities, after a baseline evaluation of the penis in the flaccid state. Measurements were recorded at 5 and 15 minutes by a certified ultrasound technician and reviewed by the principal investigator. Genital function was assessed by patient-reported genital complaints and using the validated International Prostate Symptom Score (IPSS) questionnaire. Physical function was assessed by subject-reported musculoskeletal complaints. Psychiatric and cognitive function was evaluated using the validated Patient Health Questionnaire (PHQ-9) and the Epworth Sleepiness Scale (ESS). Lastly, hypogonadal symptoms were characterized using the Androgen Deficiency in the Aging Male (ADAM) questionnaire. A positive result on the ADAM questionnaire was defined as an affirmative answer (‘‘yes’’) to questions 1 or 7 or any 3 other questions (16).
Both subjects and controls took the following validated questionnaires: IIEF, IPSS, PHQ-9, ESS and ADAM. Only subjects who had taken 5ARI underwent PDDU and were queried about genital and musculoskeletal complaints using non-validated surveys.
Baseline, demographic, laboratory and questionnaire results were extracted from medical records. Variables were tested for normal distribution using the Shapiro-Wilk test. As variables were uniformly not normally distributed, measures of central tendencies and variability were reported as median and interquartile range, respectively. Kruskal-Wallis test was used to compare categorical and ordinal variables. R 3.4.1 was used for all statistical analyses, with P<0.05 considered statistically significant.
Results
Baseline characteristics
The 5ARI group subjects had a median finasteride exposure of 18 months with an interquartile range (IQR) of 4 to 96 months. Eleven of twenty-five (44%) subjects used 1 mg of finasteride (0.2–1.25 mg). One subject used 0.5 mg of dutasteride for 24 months after discontinuation of finasteride, which he had taken for 96 months. Median total testosterone level was 450 ng/dL (IQR, 373–558 ng/dL). Median DHT value was 366 ng/dL (IQR, 373–509 ng/dL). Median age was 38 years (IQR, 33–42 years). Median body mass index (BMI) was 24.5 kg/m2 (IQR, 22.1–25.8 kg/m2). Baseline characteristics are summarized in Table 1.

Full table
Sexual function
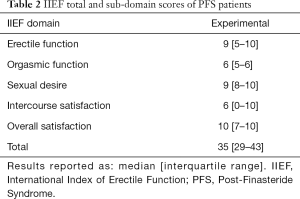
The IIEF questionnaire was completed by 23 of 25 (92%) subjects in the 5ARI group and 14 of 28 (50%) patients in the control group. A significant difference in total IIEF score between subjects in the 5ARI (median: 35; IQR: 29–43) and control group (median: 29; IQR: 27–32), P=0.035) was observed. IIEF domain scores—erectile function (EF), orgasmic function (OF), sexual desire (SD), intercourse satisfaction (IS), and overall satisfaction (OS) are summarized in Table 2. Of note, when comparing the 5ARI group to controls, there were significant differences only in the SD (9 vs. 4, P<0.001) and OS domains (10 vs. 5, P<0.001). No significant differences were noted in the erectile function (9 vs. 11, P=0.378), OF (6 vs. 6, P=0.477) and IS (6 vs. 4, P=0.164). Twenty-four of twenty-five (96%) patients in the 5ARI group underwent PDDU. In total, 17 of 25 (68%) of subjects in the 5ARI group had some vascular abnormality on penile Doppler ultrasound. Eight of twenty-four (32%) patients had arterial insufficiency [defined as peak systolic velocity <25 cm/s (17)], while 5 of 25 (20%) patients fell into the “gray zone” of possible ED, defined as a peak systolic velocity between 25–35 cm/s (17). Four of twenty-four (16%) patients had venous leak, defined as an end diastolic velocity >5 cm/s (17).
Full table
Genitourinary function
All 5ARI subjects were asked about subjective genital complaints. Nine of twenty-five (36%) subjects reported either increased descent of the testicles while the same number reported loss of penile length. Fifteen of twenty-five (60%) subjects reported some element of genital pain or numbness after starting finasteride. In total, 18 of 25 (72%) of all subjects reported at least one genital complaint.
Twenty-three of twenty-five subjects (92%) in the 5ARI arm completed the IPSS compared to 15 of 28 (54%) in the control arm. The median (IQR) for total IPSS score for the 5ARI arm was 10 [5–16] compared to 3 [2–8] for the controls (P<0.01). 5ARI subjects scored significantly worse on questions 1, 2, 5, and 8 which corresponded with incomplete emptying (P<0.01), frequent urination (P=0.02), weak stream (P=0.03), and overall quality of life (P=0.03), as demonstrated in Table 3.

Full table
Musculoskeletal effects
All subjects in the 5ARI group were queried with regards to musculoskeletal complaints, with 12 of 25 (45%) of 5ARI subjects reporting fatigue, and 5 of 25 (25%) reporting muscle atrophy. Three of 25 (12%) reported gynecomastia, weight loss, back pain, lower extremity pain and visual disturbance. Two of 25 (8%) reported weight gain, ocular hyperhidrosis, throat tightness, constipation, polydipsia, and cold flashes. One of 25 (4%) mentioned tremors, xeroderma, xerophthalmia, hypothermia, palpitations, muscle spasms, rapid aging, swollen face, and facial flushing. Overall, 19 of 25 (76%) of the subjects reported at least one musculoskeletal complaint.
Psychological and cognitive function
The 5ARI group had a higher median total PHQ-9 score when compared with the control arm [10 (6.5–16) vs. 1 (0–2), P<0.001], suggesting a higher degree of depression in the 5ARI group. Men in the 5ARI group had significantly higher scores on 8 of the 9 questions in the PHQ-9 when compared to controls (P<0.05). There was no statistically significant difference in scores between the groups in psychomotor agitation or retardation, though this did trend towards significance (P=0.073). The median total score of the 5ARI group was suggestive of moderate depressive symptoms (10–14 points). In a question evaluating the level of dysfunction in the patients’ lives due to the symptoms, the vast majority of men in the control group reported “No Difficulty At All,” while the majority of men in the 5ARI group reported symptoms making life “somewhat difficult”. Four of twenty-three men reported symptoms being “very difficult” and another 4 men reported symptoms being “extremely difficult”. These results are summarized in Table 4.

Full table
There was no difference in the median total ESS scores between the experimental (median: 5.5; IQR: 3–10.5) and control arms (median: 6; IQR: 3–8.5) (P=0.929). There was also no difference in responses to individual ESS questions.
Eighteen patients (72%) in the experimental group had a history of depression with or without anxiety documented in their chart. Two patients (8%) in the 5ARI arm committed suicide during or after the study period, whereas none of the control subjects did.
Symptoms of androgen deficiency
On the ADAM questionnaire, the 5ARI group answered yes to questions 1, 2, 3, 5, 6, 7, 10 while the control group answered yes to question 7. These results suggest that those patients in the 5ARI group were more likely to have signs and symptoms of hypogonadism. The two groups had a significant difference in scores for questions 1,2, 3, 5, 6 and 10 (all P<0.05). These were questions regarding decrease in libido, lack of energy, decrease in strength or endurance, decrease in enjoyment of life, feeling “sad” or “grumpy” and recent deterioration in work performance (all P<0.05). 5ARI subjects scored similarly to controls in questions regarding height loss, decrease in erection strength, deterioration in ability to play sports and falling asleep after dinner (P>0.05). A summary of these results is available in Table 5.

Full table
Discussion
This article sheds further light on the persistent sexual, psychological and voiding symptoms in this patient population. In addition, to our knowledge, this is the first study to present PDDU data in men who took 5ARIs for AGA. In this analysis, patients in the experimental group had a higher total IIEF score after 5ARI treatment when compared to controls. However, even though the overall IIEF score was higher, the groups had similar erectile function domain scores, which are in line with scores from patients with sexual dysfunction (18). It is noteworthy that the experimental cohort had abnormal low scores in the erectile function, OF and IS domains compared to historical IIEF controls (18). In line with this study, most of the previously published literature points to a high prevalence of sexual side effects with 5ARI administration. In a recent systematic review and meta-analysis of 17 randomized, placebo-controlled studies on the use of finasteride and dutasteride for treatment of BPH that included over 46,000 patients, these medications led to significantly higher rates of hypoactive SD and ED (6).
A considerable number of subjects had vascular abnormalities on PDDU. Some reports suggest that PDDU changes could serve as a marker of potential system wide cardiovascular changes. A recent article by Caretta et al. assessed the relationship between abnormal PDDU characteristics and rate of major adverse cardiovascular events (19). Although the authors found that abnormal Peak Systolic Velocity (PSV <30 cm/s) values were not significantly associated with increased risk of cardiovascular events, the authors did note an increase in cardiovascular events (RR 3.2, 95% CI: 1.2–8.8) in subjects with abnormal cavernosal artery morphology (intima media thickness >0.4 mm). This association was significant even when controlling for baseline risk factors including age, blood sugar level, total cholesterol, hypertension and smoking status. Further studies should include measurements of a penile plaque and arterial morphological characteristics in subjects with 5ARI exposure, as these may provide a more complete picture of penile vascular physiology in patients exposed to 5ARI.
Subjects in the 5ARI group had higher IPSS scores than controls. BPH is increasingly being thought of as an immune modulated condition, with studies showing a significant relationship between the level of lower urinary tract symptoms (LUTS) and markers of chronic inflammation (20). Although inhibition of 5α-reductase is a primary mechanism of treatment, the low DHT environment associated with finasteride may potentiate inflammation, and paradoxically worsen BPH symptoms. Recent studies have shown that a low DHT environment in the prostate can recruit CD8 T cells to prostatic epithelial cells (21). Follow up studies have shown that these CD8 cells can induce further proliferation of these epithelial cells through CCL5/STAT1/CCND1 signaling pathways (22). This proliferation, thought to be the cause of therapeutic failure after finasteride treatment for BPH, may also provide an explanation of our findings. 5ARIs may not have had a treatment effect on lower urinary tract symptoms (LUTS) in our patient cohort as these are younger men and likely to have prostates significantly smaller than 40 cc (23).
The musculoskeletal domain results we observed were in line with or more significant than those in other studies. Our study subjects reported a 12% prevalence of gynecomastia, which is higher than the 1–5% reported in other studies (24,25), but closer to self-reported patient accounts (26). The reported 20% muscle atrophy rate was higher than the self-reported rate of 5.8–9.3% in the Food and Drug Administration Adverse Event Reporting System Database (FAERS) (26). Eight percent of our patient cohort reported weight gain, similar to the 9.4% reported in FAERS (26). Although not quantified in the general population, complaints regarding loss of genital sensation or change in genital appearance associated with finasteride use are becoming increasingly well documented in the literature. Multiple studies have shown the percentage of men reporting genital sensory changes to be anywhere from 79–87% (9,10) after finasteride use, consistent with the 72% of men in the 5ARI group of our study.
Subjects in the 5ARI group reported more significant psychological and cognitive symptoms, measured using the PHQ-9, than controls. The median PHQ-9 score fell in the moderate depressive symptoms range. Previously, Basaria et al. found that symptomatic finasteride users (n=25) fell into the moderate depression range (27). In the same study, the PHQ-9 scores were independent of finasteride treatment duration (P=0.406) or time elapsed since ceasing finasteride treatment (P=0.180). Basaria et al. also showed that when compared to asymptomatic finasteride users and healthy men, PFS patients had higher depression scores on the Beck Depression Inventory (P<0.001) and Hamilton Depression Inventory (overall P<0.001).
Results of the PHQ-9 in this study showed that patients in the PFS group reported high levels of anhedonia and feelings of hopelessness, which is in line with prior work. Other studies have found 30-73% of PFS patients reporting these symptoms (9,11). Ganzer and Jacobs found that 55% of patients with a history of finasteride use for AGA had a previous Axis I or II psychiatric diagnosis and 28.8% had a positive family history of a psychiatric illness in a first-degree relative (25). The findings of both this study and Ganzer and Jacobs (25) are confounded by the higher rates of suicidal ideation in patients with AGA (28). Given that two subjects committed suicide during or after this study, psychiatric side effects and the risks of finasteride use in patients with a history of psychiatric disorders should be investigated further. At this time, patients should undergo extensive counseling regarding sexual and non-sexual side effects that may occur after both initiation and discontinuation of this medication class.
A significant number of subjects self-reported cognitive symptoms including forgetfulness and brain fog. As in our study, Ganzer et al. (25) found high rates of mental cloudiness (75%), slowed thought process (74%), and attentional difficulties (74%) in men responding to an internet survey regarding the side effects of finasteride. Basaria et al. used validated objective tests to evaluate men that reported cognitive side effects after stopping finasteride (27). The authors found no impaired performance on cognitive function and memory tests (P>0.10). As there are a significant number of men reporting cognitive symptoms after stopping 5ARIs, the discrepancy between the self-reported symptoms and objective evaluations should be investigated further.
The median total ESS scores of the 5ARI arm fell below the common cutoff for excessive daytime sleepiness of >10. However, Sanford et al. have suggested that the ESS score threshold of >10 may fail to accurately capture the number of patients experiencing significant sleep disturbances (29).
Interestingly, in an analysis of the FAERS database, Gupta et al. found an increased rate of developing obstructive sleep apnea following finasteride use when compared to other medications (30). Given this as well as the higher number of sleep disturbances reported by the PFS arm on the PHQ-9, abnormal sleep findings after finasteride use should be the focus of future studies.
Both 5ARI and control groups provided positive answers to the ADAM questionnaire. The former reported yes on questions 1 and 7 and 2, 3, 5, 6, 7, 10 while the latter reported yes to question 7. Question 7 addressed erection strength and as reported above, may have been impacted by a small difference in age or possible differences in baseline erections and is consistent with our IIEF results. Of note, differences were seen in the questions regarding decreased enjoyment in life and feeling “sad” or “grumpy”, which are in line with the differences found in the PHQ-9 questionnaire. In addition, the rates of decline in athletic ability were similar between the 5ARI and control arms, suggesting a minimal decline in functional strength. In line with our musculoskeletal survey results, Ganzer et al. reported high rates of muscle weakness (56%), increased fat deposition (54%), and muscle twitching (47%) in PFS patients (9). On the contrary, Basaria et al. found no difference in trunk to limb fat ratio, leg press strength, or lean and fat mass in the trunk, limbs, and whole-body between PFS patients, former finasteride users without PFS, and healthy controls (27). The discrepancy between self-reported symptoms and physical evaluation should be further investigated.
There are several strengths and weaknesses of the present study that deserve mention. Strengths include the use of validated questionnaires, PDDU data, hormonal laboratory values, and baseline psychiatric assessments in all subjects. Limitations include inconsistency in survey completion rates between groups, variable duration of 5ARI therapy, selection bias, lack of baseline questionnaire data before 5ARI use, and lack of PDDU results in the control arm.
Conclusions
The present study supports the conclusion that 5ARI use may predispose to persistent sexual, genitourinary, psycho-cognitive, and anti-androgenic changes even after 5ARI therapy is discontinued. Furthermore, this is the first study to present data on PDDU results in patients who took 5ARIs for AGA. As the FDA amended the package insert to include a warning of sexual dysfunction that persists after stopping finasteride, we recommend additional clinical studies that assess non-sexual side effects that may persist after discontinuation of the drug. Given the significance of the observed and potential side effects, patients should be extensively counseled and monitored for possible side effects after initiation and discontinuation of this medication class.
Acknowledgments
Funding: This study was funded by an unrestricted grant from the Post-Finasteride Foundation. AP, MD, PhD is a National Institutes of Health K08 Scholar supported by a Mentored Career Development Award (K08DK115835-01) from the National Institute of Diabetes and Digestive and Kidney Diseases. This work is also supported in part through a Urology Care Foundation Rising Stars in Urology Award (to AP).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.03.21). MK is a consultant for Endo, AbbVie, and Boston Scientific. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Baylor College of Medicine and informed consent was taken from all the patients prior to the start of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- U.S. Food and Drug Administration. 5-Alpha Reductase Inhibitor Information [Internet] 2016. Available online: https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm258424.htm
- Finasteride Tablets Full Prescribing Information Indications and Usage. Whitehouse Station, New Jersey: Merck Sharp & Dohme Corporation, 2012:1-18.
- Post Finasteride Syndrome Foundation Overview [Internet] 2018. Available online: https://www.pfsfoundation.org/abount-post-finasteride-syndrome-foundation/
- US Department of Health and Human Services. Adverse events of 5-alpha-reductase inhibitors [Internet] 2016. Available online: https://rarediseases.info.nih.gov/diseases/12407/adverse-events-of-5-alpha-reductase-inhibitors
- Than J, Rodriguez K, Khera M. Post-finasteride Syndrome: A Review of Current Literature. Curr Sex Heal Reports 2018;10:152-7. [Crossref]
- Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5 a - reductase for benign prostatic hyperplasia : a comprehensive review and meta-analysis. Andrology 2017;5:671-8. [Crossref] [PubMed]
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5 a -reductase inhibitors, finasteride, or dutasteride. Peer J 2017;5:e3020. [Crossref] [PubMed]
- Irwig MS. Persistent Sexual Side Effects of Finasteride : Could They be Permanent? J Sex Med 2012;9:2927-32. [Crossref] [PubMed]
- Ganzer CA, Jacobs AR, Iqbal F. Persistent Sexual, Emotional, and Cognitive Impairment Post-Finasteride : A Survey of Men Reporting Symptoms. Am J Mens Health 2015;9:222-8. [Crossref] [PubMed]
- Chiriacò G, Cauci S, Mazzon G, et al. An observational retrospective evaluation of 79 young men with long-term adverse effects after use of finasteride against androgenetic alopecia. Andrology 2016;4:245-50. [Crossref] [PubMed]
- Walf AA, Kaurejo S, Frye CA. Research Brief : Self-Reports of a Constellation of Persistent Antiandrogenic, Estrogenic, Physical, and Psychological Effects of Finasteride Usage Among Men. Am J Mens Health 2018;12:900-6. [Crossref] [PubMed]
- Andersen JT, Curtis Nickel J, Marshall VR, et al. Finasteride significantly reduces acute urinary retention and need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology 1997;49:839-45. [Crossref] [PubMed]
- Bartsch G, Rittmaster R, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol 2002;19:413-25. [Crossref] [PubMed]
- Traish AM, Hassani J, Guay AT, et al. Adverse Side Effects of 5a-Reductase Inhibitors Therapy: Persistent Diminished Libido and Erectile Dysfunction and Depression in a Subset of Patients. J Sex Med 2011;8:872-84. [Crossref] [PubMed]
- Giatti S, Diviccaro S, Panzica G, et al. Post-finasteride syndrome and post-SSRI sexual dysfunction: two sides of the same coin? Endocrine 2018;61:180-93. [Crossref] [PubMed]
- Morley JE, Charlton E, Patrick P, et al. Validation of a Screening Questionnaire for Androgen Deficiency in Aging Males. Metabolism 2000;49:1239-42. [Crossref] [PubMed]
- Wilkins CJ, Sriprasad S, Sidhu PS. Colour Doppler Ultrasound of the Penis. Clin Radiol 2003;58:514-23. [Crossref] [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [Crossref] [PubMed]
- Caretta N, Ponce MDR, Minicuci N, et al. Penile doppler ultrasound predicts cardiovascular events in men with erectile dysfunction. Andrology 2019;7:82-7. [Crossref] [PubMed]
- Nickel JC, Roehrborn CG, O’Leary MP, et al. The Relationship between Prostate Inflammation and Lower Urinary Tract Symptoms: Examination of Baseline Data from the REDUCE Trial. Eur Urol 2008;54:1379-84. [Crossref] [PubMed]
- Fan Y, Hu S, Liu J, et al. Low intraprostatic DHT promotes the infiltration of CD8+ T cells in BPH tissues via modulation of CCL5 secretion. Mediators Inflamm 2014;2014.
- Yang Y, Hu S, Liu J, et al. CD8+ T cells promote proliferation of benign prostatic hyperplasia epithelial cells under low androgen level via modulation of CCL5/STAT5/CCND1 signaling pathway. Sci Rep 2017;7:42893. [Crossref] [PubMed]
- Roehrborn CG, Boyle P, Bergner D, et al. Serum prostate-specific antigen and prostate volume predict long-term changes in symptoms and flow rate: results of a four-year, randomized trial comparing finasteride versus placebo. PLESS Study Group. Urology 1999;54:662-9. [Crossref] [PubMed]
- Kaplan SA, Chung DE, Lee RK, et al. A 5-year retrospective analysis of 5 a-reductase inhibitors in men with benign prostatic hyperplasia : finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteri. Int J Clin Pract 2012;66:1052-5. [Crossref] [PubMed]
- Ganzer CA, Jacobs AR. Emotional Consequences of Finasteride : Fool’s Gold. Am J Mens Health 2018;12:90-5. [Crossref] [PubMed]
- Baas WR, Butcher M, Holland B, et al. A Review of the FAERS Data on 5-Alpha Reductase Inhibitors: Implications for Post-Finasteride Syndrome. Urology 2018;120:143-9. [Crossref] [PubMed]
- Basaria S, Jasuja R, Huang G, et al. Characteristics of Men Who Report Persistent Sexual Symptoms After Finasteride Use for Hair Loss. J Clin Endocrinol Metab 2016;101:4669-80. [Crossref] [PubMed]
- Muscarella F, Cunningham MR. The Evolutionary Significance and Social Perception of Male Pattern Baldness and Facial Hair. Ethol Sociobiol 1996;17:99-117. [Crossref]
- Sanford SD, Lichstein KL, Durrence HH, et al. The influence of age, gender, ethnicity, and insomnia on Epworth sleepiness scores: a normative US population. Sleep Med 2006;7:319-26. [Crossref] [PubMed]
- Gupta M, Vujcic B, Sheridan A, et al. Finasteride is Associated with a Higher Odds of Obstructive Sleep Apnea (OSA): Results from the US FDA Adverse Events Reporting System (FAERS). Sleep 2018;41:340-1. [Crossref]


