
Technical management of inguinal lymph-nodes in penile cancer: open versus minimal invasive
Introduction
Penile cancer is rare malignancy in the US, which accounts for less than 1% of all malignant neoplasms in men, while it represents up 6% of male malignancies in several developing nations of Asia, Africa, and South America (1). This discrepancy in incidence is mostly related to low rates of neonatal circumcision in such nations, which is known to be protective factor for the disease (2). The management of penile cancer is surgical, with organ sparing techniques becoming increasingly popular due to the disfiguring nature and psychological distress caused by historical procedures (3). Following complete excision of the penile cancer, bilateral inguinal lymphadenectomy (ILND) is the standard of care for patient with intermediate and high-risk penile cancer (≥pT1bN0 or pTanyN1-2) (4). Performance of ILND is not only imperative for disease staging (4), but early ILND has been shown to offer a survival advantage (5). Penile cancer remains one of the few disease processes, along with testis cancer (6), in which a modifiable factor, such as lymphadenectomy, has been proven to provide a survival advantage.
Utilization of ILND for penile cancer patients in the US remains low, with reported rates ranging from 19.6% to 27.2% (7-9). Marginal ILND utilization is not specific to penile cancer, with vulvar cancer (20.4%) (10) and lower extremity melanoma (39%) (11) reporting comparable rates. Avoidance of ILND utilization points into a technique-specific issue rather than disease biology. In recent review of the NCDB database, ILND was more likely to occur in younger patients, those with a more contemporary diagnosis, and those treated in an Academic/Research facility (7). Interestingly, insurance type, household income, and demographic location had no effect on lymphadenectomy utilization, suggesting, that discrepancies in ILND utilization are likely related to physician experience and comfort rather patient factors.
The main hesitancy for the use of ILND is the significant comorbidity associated with the procedure, which has a reported complication rate of 14–37% (12-16). As a result, several technique modifications have been proposed to the historical open radical ILND with the purpose to minimize wound complications and the development of chronic lower extremity lymphedema. The increasing popularity of minimally invasive procedures and introduction of robotic surgery have led to a rapid development of minimally invasive surgery (MIS) and robotic techniques for the performance of the ILND with a proposed reduction in postoperative complication rates. Here we review the available surgical techniques for ILND: open, laparoscopic, and robotic and discuss their oncological efficacy and associated surgical morbidity.
Open ILND
Lymphatic spread in penile cancer
The lymphatic drainage to the penis has been closely studied (17), and it follows a specific spread pattern consisting of superficial inguinal nodes -> deep inguinal nodes -> pelvic nodes -> retroperitoneal nodes. The penis being a central organ, lymphatic spread seldom follows laterality, with bilateral nodal spread seen in up to 81% of patients (18,19). The inguinal region is divided into the five zones (20), by a horizontal and verticals lines centered at the fossa ovalis. Historically, the teaching has been to resect the nodal tissue in all five zones, which has been associated with significant postoperative complications (wound) and morbidity (lymphedema) (21). As a result, the radical lymphadenectomy has been modified by limiting the nodal dissection (16,22) while balancing appropriate oncological outcomes.
Radical ILND
The radical dissection of the inguinal region (21) consists on dissection of all nodal tissue limited by the anterior superior iliac spine (ASIS) superior laterally, the pubic tubercle superior medially, the abductor longus muscle medially and the sartorius muscle laterally. The greater saphenous vein is ligated, and nodal tissue over the femoral vessels is excised (20). The radical lymphadenectomy leaves the femoral triangle exposed which may lead to possible vessel erosion if a wound infection is to occur. Historically, a sartorius muscle interposition was performed for coverage for the exposed triangle (21), but recent studies have shown that the sartorius interposition may provide limited coverage and more robust rotational flaps (tensor fascial lata, gracilis, anterolateral thigh, internal oblique and vertical rectus abdominis) are currently favored (23).
Although the radical ILND is considered the oncological gold standard, the technique is associated with significant postoperative morbidity (Table 1). The largest reported series of radical ILND (12), reported post-operative complication rate of 61.7%, with most of the complications being wound-related (infection, dehiscence, and necrosis). Contemporary series (Table 1) have reduced the wound complication significantly with careful creation and manipulation of skin flaps (preserving Scarpa’s fascia), preservation of the skin perforating vessels, and control of postoperative seromas with closed suction surgical drains (13-15).
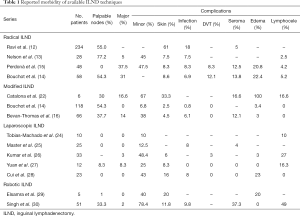
Full table
Modified ILND
As a result of the high morbidity associated with the radical ILND, Catalona et al. proposed a modified lymphadenectomy technique (22), which aimed to preserve oncological benefit while reducing the postoperative morbidity. The technique limited the size of the incision, excluded nodal dissection lateral to the femoral artery and caudal to the fossa ovalis, while preserving the greater saphenous vein, and eliminating the sartorius transposition (22). The modified lymphadenectomy significantly decreased the incidence of early (6.8% vs. 41.1%) and late (3.4% vs. 43.1%) surgical complications (Table 1) compared to the radical dissection (14). Consequently, the Catalona modified technique was rapidly and widely adopted for patients with intermediate (pT1b) and high risk (≥pT2) penile cancer with no clinical evidence of nodal metastases. Lopes and colleagues (31), warned regarding a potential understaging and under-treatment with the Catalona modified technique (22), reporting an in-field recurrence in 17% due to the avoidance of the lateral and central dissection fields. As a result, a contemporary limited ILND has been proposed in which the boundaries of dissection are expanded medially and laterally to include the midpoint of the adductor longus and the lateral boundary of the sartorius muscle (16). Furthermore, frozen section of clinically prominent nodes should be highly consider to assess the need for a more extended dissection (deep inguinal nodes) (16).
Minimally invasive approaches
In order to minimize morbidity associated with open ILND, introduction of minimally invasive techniques (MIS) have been proposed, which would limit the incision size and the need for flap creation. Bishoff and colleagues (32,33) were the first to introduce the idea of a minimally invasive ILND, utilizing an endoscopic approach, developed in cadaveric studies and then performed in patient with promising results. Since several MIS techniques have been proposed: endoscopic subcutaneous modified ILND (ESMIL) (34), video-endoscopic ILND (VEIL) (24), and the leg endoscopic groin lymphadenectomy (LEG) (25), VEIL with saphenous sparing (28,35), single-site VEIL (SSVEIL) (27,36). The techniques all accomplish the same goal but differ slightly, with the VEIL and ESMIL techniques limited to only removing the superficial nodes while the LEG technique reports a complete lymphadenectomy (superficial and deep nodes). Further optimization of the technique with saphenous sparing procedure (28,35) and single-site laparoscopy (27) are being reported with promising results.
Endoscopic ILND is approached in a retrograde fashion by placing the access ports in the inferior aspect of the femoral triangle. The inferior border to the femoral triangle is identified by measuring 20 cm below the ASIS and 15 cm from the pubic tubercle and connecting the lines between the two (Figure 1A,B). The camera port is placed in the middle of the connecting incision and the skin flap is then created either with blunt finger dissection or a dissecting laparoscopic access balloon. Two approaches to the lymphadenectomy have been reported: a superficial to deep approach or a deep to superficial dissection (Figure 2A,B). In the superficial to deep dissection, the working space is created between Scarpa’s fascia and the nodal packet, and the lymphadenectomy is performed from a superficial to deep approach. In the deep to superficial approach, the working space is created between the fascia Latta and the nodal packet. The dissection is then carried by releasing the nodal packet from Scarpa’s fascia. In our experience, the saphenous vein is more readily to identified with the deep to superficial approach, which is important if a saphenous sparing procedure is planned. There is no report comparing either approach in regard to incidence of complications or oncological outcomes.
Level I evidence on the oncological effectiveness of MIS remains lacking, mainly due to the rarity of the disease. Tobias-Machado et al., at Emory University, opened a trial aiming to randomized patients to the MIS vs. the open technique, but the trial closed due to poor accrual. The available data relies on retrospective series and prospective studies comparing lymph node yields. Tobias-Machado and colleagues (37), reported the first comparison between the VEIL and open ILND were patients underwent the endoscopic approach (VEIL) in one limb and the standard ILND in the other. They reported comparable node yields between techniques, with no difference in recurrence patterns. In a series by Kumar and colleagues (26), the results of 33 consecutive VIEL procedures was compared to 35 open ILND, which showed a superior lymph node yield with the VEIL technique (9.36 vs. 7.11; P=0.012), and no inguinal recurrences at the median follow-up of 16 months.
The introduction of endoscopic procedures has demonstrated a clear advantage in reducing the morbidity associated with ILND (Table 1). The landmark study by Tobias-Machado et al. (24), noted a significant reduction in overall complications with the VEIL procedure (20% vs. 70%, P=0.015) compared to the open approach. In a follow-up study by Master et al., in which the long-term outcomes were evaluated, the VEIL procedure was associated with minimal post-operative complicated with only one case of developing flap necrosis (2.6%), and 5 cases of wound seroma (12%). Cui et al. (28) evaluated the benefit of saphenous vein preservation during laparoscopic ILND, by performing a saphenous sparing procedure in one groin while ligating the vein in the other. The study included 23 patients, and saphenous preservation was associated with a significant reduction in the incidence of acute and long-term lymphedema, with no difference on lymph node yield. Yuan et al. (27) compared the use of single-site VEIL to standard VEIL, with emphasis in saphenous preservation, noting no difference in operative time, lymph node yield or surgical complications. The authors did report improve patient satisfaction scores in the single-site group (75% vs. 25%; P=0.039) in cosmetic results.
A prospective study Jakub and colleagues (38), aimed to assess the reproducibility and learning curve associated with endoscopic ILND. The study consisted of a structured didactic program combined with a hands-on session would prepare high volume melanoma surgeons to perform a MIS ILND safely and proficiently. The study showed that most surgeon were deemed proficient within 6 cases (83%), with conversion rates reduced significantly after the 5th case. The learning curve associated with this procedure is of significant importance due to the oncological implications associated with the procedure. Surgeons adopting an MSI approach should carefully balance oncological quality of the procedure with the post-operative benefits for an MIS approach, and conversion should be considered if an inadequate lymphadenectomy is at risk.
Robotic-assisted VEIL (RAVEIL)
As it has occurred for the many of established laparoscopic procedures, the transition to the robotic-assisted laparoscopic platform was expected, due to the improved visualization provided by the three-dimensional optics, along with the improved dexterity over the laparoscopic instruments.
The first robotic ILND was performed by Josephson et al., who described the procedure in one patient (39). Matin and colleagues at the MD Anderson Cancer Center (40) set to proved the oncological efficacy of the procedure by performing a prospective trial in which an open inguinal exploration evaluated the robotic lymphadenectomy. The study consisted of 10 patients with invasive penile cancer, who underwent a RAVEIL followed by an open inguinal exploration by an experienced open surgeon performed, noting an adequate lymphadenectomy in 94.7% of inguinal fields (40).
Several retrospective case reports and series (29,39,41) have been published discussing the feasibility of the RAVEIL and its associated complication rate. Singh et al. (30), provide the largest retrospective series in which 51 patients treated with RAVEIL technique were compared to 100 patients who underwent open lymphadenectomy. The RAVEIL technique was associated with shorter hospital stay and decreased postoperative complications (wound/flap necrosis and lower extremity edema) while achieving comparable node yields (30). The majority of patients undergoing RAVEIL had clinically N0 disease (67%), with only 4% harboring locally advance disease at the time of excision (30). On multivariate analysis, increasing pathological nodal stage [OR 2.8 (95% CI: 1.1–6.8), P=0.027] and undergoing an open lymphadenectomy [OR 7.5 (95% CI: 1.3–43), P=0.024] were predictive factors associated with a major postoperative complication (30).
Laparoscopic access for the RAVEIL technique is similar to that of the VEIL technique, with minor difference that an adequate distance of 6–8 cm between the robotic ports is necessary to minimize collisions (Figure 1B). One of the main limitations of the RAVEIL, is the need for the repositioning of the robot to the contralateral side if bilateral dissections are to occur during a single anesthetic. Sotelo and colleagues (41), recently reported their robotic docking technique in which the robot does not need to be moved to the contralateral side of the patient, just repositioned. In their technique, the robot is docked at a 45-degree angle for the contralateral thigh and then repositioned parallel to the patient for the ipsilateral thigh. The authors report minimal collisions with the proposed docking technique.
Limited reports have evaluated the learning curve associated with the RAVEIL technique. Elsamra et al. (29), an experienced robotic surgeon, provided the only account of a potential learning curve associated with the procedure. In his report, he noted that an appropriate lymph-node yield (n>7) was only achieved after the third patient. He reported a single conversion in a patient with bulky inguinal adenopathy and recommended the procedure be limited to patients with clinically node-negative disease (cN0).
Conclusions
ILND in patients with invasive penile cancer has been associated with superior oncological outcomes with patients with invasive disease. Unfortunately, ILND remains underutilized due to its associated morbidity, with only a third of eligible patients receiving the procedure in the US. Several open and MIS techniques have been introduced over the last 30 years hoping to decrease the associated morbidity while maintaining the oncological efficacy. As a result, the modified open ILND has become the standard of care for prophylactic lymphadenectomies, while the radical lymphadenectomy continues to be recommended for those with palpable and locally invasive disease. Both laparoscopic and robotic techniques have been introduced to further minimize wound-related complications (infection, flap necrosis, and dehiscence) associated with the open technique and are gaining popularity. As the field moves towards the adoption of endoscopic techniques (laparoscopic or robotic), one must remember that the oncological data available for these techniques remains retrospective and associated with uncontrolled bias. Moreover, the majority of the endoscopic reports have focused on clinically node-negative disease, with seldom reports including patients with node positive disease. Lastly, there is a demonstrated learning curve associated with both laparoscopic and robotic procedures, which need to be considered when adopting these endoscopic techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marc C. Smaldone and Jeffrey J. Tomaszewski) for the series “Controversies in Minimally Invasive Urologic Oncology” published in Translational Andrology and Urology. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.04.02). The series “Controversies in Minimally Invasive Urologic Oncology” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Douglawi A, Masterson TA. Updates on the epidemiology and risk factors for penile cancer. Transl Androl Urol 2017;6:785-90. [Crossref] [PubMed]
- Pow-Sang MR, Ferreira U, Pow-Sang JM, et al. Epidemiology and natural history of penile cancer. Urology 2010;76:S2-6. [Crossref] [PubMed]
- Azizi M, Chipollini J, Peyton CC, et al. Current controversies and developments on the role of lymphadenectomy for penile cancer. Urol Oncol 2019;37:201-8. [Crossref] [PubMed]
- Clark PE, Spiess PE, Agarwal N, et al. Penile cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw 2013;11:594-615. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol 2005;173:816-9. [Crossref] [PubMed]
- Stephenson AJ, Sheinfeld J. Management of patients with low-stage nonseminomatous germ cell testicular cancer. Curr Treat Options Oncol 2005;6:367-77. [Crossref] [PubMed]
- Correa AF, Handorf E, Joshi SS, et al. Differences in survival associated with performance of lymph node dissection in patients with invasive penile cancer: results from the National Cancer Database. J Urol 2018;199:1238-44. [Crossref] [PubMed]
- Chipollini J, Tang DH, Sharma P, et al. Patterns of regional lymphadenectomy for clinically node-negative patients with penile carcinoma: analysis from the national cancer database from 1998 to 2012. Clin Genitourin Cancer 2017;15:670-7.e1. [Crossref] [PubMed]
- Johnson TV, Hsiao W, Delman KA, et al. Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients: results from the Surveillance, Epidemiology, and End Results program. Cancer 2010;116:2960-6. [Crossref] [PubMed]
- Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulvar cancer in the United States. Gynecol Oncol 2008;108:577-83. [Crossref] [PubMed]
- Bilimoria KY, Balch CM, Bentrem DJ, et al. Complete lymph node dissection for sentinel node-positive melanoma: assessment of practice patterns in the United States. Ann Surg Oncol 2008;15:1566-76. [Crossref] [PubMed]
- Ravi R. Morbidity following groin dissection for penile carcinoma. Br J Urol 1993;72:941-5. [Crossref] [PubMed]
- Nelson BA, Cookson MS, Smith JA Jr, et al. Complications of inguinal and pelvic lymphadenectomy for squamous cell carcinoma of the penis: a contemporary series. J Urol 2004;172:494-7. [Crossref] [PubMed]
- Bouchot O, Rigaud J, Maillet F, et al. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol 2004;45:761-5; discussion 765-6. [Crossref] [PubMed]
- Perdonà S, Autorino R, De Sio M, et al. Dynamic sentinel node biopsy in clinically node-negative penile cancer versus radical inguinal lymphadenectomy: a comparative study. Urology 2005;66:1282-6. [Crossref] [PubMed]
- Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. J Urol 2002;167:1638-42. [Crossref] [PubMed]
- Horenblas S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int 2001;88:473-83. [Crossref] [PubMed]
- Horenblas S, Jansen L, Meinhardt W, et al. Detection of occult metastasis in squamous cell carcinoma of the penis using a dynamic sentinel node procedure. J Urol 2000;163:100-4. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Meinhardt W, et al. Dynamic sentinel node biopsy in penile carcinoma: evaluation of 10 years experience. Eur Urol 2005;47:601-6; discussion 606. [Crossref] [PubMed]
- Daseler EH, Anson BJ, Reimann AF. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg Gynecol Obstet 1948;87:679-94. [PubMed]
- Spratt J. Groin dissection. J Surg Oncol 2000;73:243-62. [Crossref] [PubMed]
- Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J Urol 1988;140:306-10. [Crossref] [PubMed]
- Murthy V, Gopinath KS. Reconstruction of groin defects following radical inguinal lymphadenectomy: an evidence based review. Indian J Surg Oncol 2012;3:130-8. [Crossref] [PubMed]
- Tobias-Machado M, Tavares A, Molina WR Jr, et al. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int Braz J Urol 2006;32:316-21. [Crossref] [PubMed]
- Master V, Ogan K, Kooby D, et al. Leg endoscopic groin lymphadenectomy (LEG procedure): step-by-step approach to a straightforward technique. Eur Urol 2009;56:821-8. [Crossref] [PubMed]
- Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int 2017;119:530-4. [Crossref] [PubMed]
- Yuan JB, Chen MF, Qi L, et al. Preservation of the saphenous vein during laparoendoscopic single-site inguinal lymphadenectomy: comparison with the conventional laparoscopic technique. BJU Int 2015;115:613-8. [Crossref] [PubMed]
- Cui Y, Chen H, Liu L, et al. Saphenous vein sparing during laparoscopic bilateral inguinal lymphadenectomy for penile carcinoma patients. Int Urol Nephrol 2016;48:363-6. [Crossref] [PubMed]
- Elsamra SE, Poch MA. Robotic inguinal lymphadenectomy for penile cancer: the why, how, and what. Transl Androl Urol 2017;6:826-32. [Crossref] [PubMed]
- Singh A, Jaipuria J, Goel A, et al. Comparing outcomes of robotic and open inguinal lymph node dissection in patients with carcinoma of the penis. J Urol 2018;199:1518-25. [Crossref] [PubMed]
- Lopes A, Rossi BM, Fonseca FP, et al. Unreliability of modified inguinal lymphadenectomy for clinical staging of penile carcinoma. Cancer 1996;77:2099-102. [Crossref] [PubMed]
- Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003;170:2217-20. [Crossref] [PubMed]
- Bishoff JT, Basler JW, Teichman JM, et al. Endoscopic subcutaneous modified inguinal lymph node dissection (ESMIL) for squamous cell carcinoma of the penis. J Urol 2003;169:78.
- Alvarez-Maestro M, Rios Gonzalez E, Martinez-Pineiro L, et al. Modified endoscopic left inguinal lymphadenectomy. Actas Urol Esp 2013;37:663-6. [Crossref] [PubMed]
- Chiapparrone G, Rapisarda S, de Concilio B, et al. Saphenous-sparing laparoscopic inguinal lymphadenectomy. Int Braz J Urol 2018;44:645-6. [Crossref] [PubMed]
- Tobias-Machado M, Correa WF, Reis LO, et al. Single-site video endoscopic inguinal lymphadenectomy: initial report. J Endourol 2011;25:607-10. [Crossref] [PubMed]
- Tobias-Machado M, Tavares A, Ornellas AA, et al. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol 2007;177:953-7; discussion 958. [Crossref] [PubMed]
- Jakub JW, Terando AM, Sarnaik A, et al. Training high-volume melanoma surgeons to perform a novel minimally invasive inguinal lymphadenectomy: report of a prospective multi-institutional trial. J Am Coll Surg 2016;222:253-60. [Crossref] [PubMed]
- Josephson DY, Jacobsohn KM, Link BA, et al. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 2009;73:167-70; discussion 170-1. [Crossref] [PubMed]
- Matin SF, Cormier JN, Ward JF, et al. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 2013;111:1068-74. [Crossref] [PubMed]
- Sotelo R, Cabrera M, Carmona O, et al. Robotic bilateral inguinal lymphadenectomy in penile cancer, development of a technique without robot repositioning: a case report. Ecancermedicalscience 2013;7:356. [PubMed]




