
Transvaginal management of symptomatic complex urethral diverticula by definite closure of diverticula and robust reconstruction of the urethra
Introduction
Urethral diverticula (UDs) have been reported to affect 20 out of 1,000,000 adult women per year (1). However, it is widely believed that the nonspecific and varied symptoms of UDs lead to under-reporting of the true data. The classic presentations of UDs are described as “3Ds” (dysuria, postvoid dribbling, and dyspareunia) (2). While UD patients are more likely to suffer from lower urinary tract symptoms (LUTS), recurrent urinary infection, and stress urinary incontinence (2). According to the published literatures, UDs are usually diagnosed after consulting with an average of 9 physicians within 5.2 years (3). Therefore, accurate diagnosis of UDs requires detailed disease history, physical examination, urethrovesical endoscopic examination, and targeted radiologic imaging (4).
According to the anatomic configuration, UDs are classified into simple, saddle shaped and circumferential types (5). The saddle shaped, multiloculated, and circumferential types are also regarded as complex UDs, which increase difficulties of surgical treatment (5,6). Management of complex UDs is challenging not only for the ostia detection and urethral reconstruction during surgery but also for the high risk of postoperative complications (6). Preoperative acknowledgment of the anatomic configuration and selection of an appropriate surgical approach for UDs could reduce surgical difficulties and postoperative complications (4).
Several surgical approaches for UDs have been described (7). Transvaginal diverticulectomy is highly effective for symptomatic UDs with reported cure rate of more than 70% (8). Nickles et al. also reported suprameatal incision for anterior horseshoe-shaped UDs (6). Complete dissection of UDs is the classic surgical method, which brings extensive destruction to the urethra. And Martius flap interposition is selectively utilized because of extensive urethral defect after total diverticulectomy (6,9). Meanwhile, there are several surgical modalities concerning partial diverticulectomy, including endoscopic unroofing, fulguration, and transvaginal marsupialization (9-11). However, the above surgical methods of partial resection are limited by the applicability and fluctuated success rates (9-11).
In the present study, we would introduce a modified surgical technique of partial diverticulectomy for symptomatic complex UDs. After accurate locating the diverticular neck, we focused on definite closure of the diverticular ostia and robust urethral reconstruction, which contributed to continent function and low postoperative complications. This is the first report of surgical technique for management of symptomatic complex UDs in China. We present the following article in accordance with the STROBE Guideline.
Methods
Data source
The database of medical record library was retrospectively searched for 39 patients who underwent partial diverticulectomy for symptomatic and complex UDs from January 2002 to December 2018. Patients with simple UDs were excluded. We collected medical information including disease history, voiding diary, physical examination, transperineal ultrasound, cross-sectional postvoiding magnetic resonance imaging (MRI), and cystourethroscopy. The LNS C3 classification was used to describe UDs (12). Each letter of the “LNS C3” represents a characteristic of UDs: L describes location, N describes number, S describes size, and C3 describes configuration, communication, and continence. All surgical procedures were performed by the two surgeons (SW and YY). The surgical outcomes were assessed by overall operative time, estimated blood loss, hospitalization stay, surgical complications, and follow-up outcomes. This study was approved by the Peking University First Hospital review board. Informed consent was obtained from all participants.
Surgical technique
Patients were placed in lithotomy position in combination with slight trendelenburg position. First, the cystourethroscopy was performed to determine the distance between the bladder neck and the UD. Meanwhile, the location of a UD on the urethra was also recorded (Figure 1A). Usually, ostia of UDs could be detected at 5 o’clock and/or 7 o’clock of midpiece of the urethra by endoscopy (13). In this part, we used a 12-degree rigid nephroscope with the water outlet at its front tip to examine the whole urethra. Therefore, the ostia would be opened and the diverticulum would be filled when the nephroscope was nearby, which facilitated detection of UDs. A urethral catheter was placed.
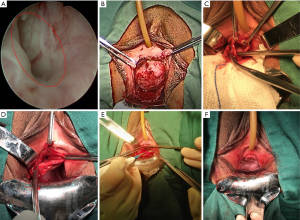
The surgeon infiltrated approximate 50 mL saline solution into the space between vagina and urethra to reduce bleeding. Then, an inverted U-shaped flap in full-thickness was separated from the anterior vaginal wall to expose the UD (Figure 1B). Of note, periurethral fibromuscular tissue was not essential to be absolutely mobilized away, especially on lateral sides. We used low energy electrocution (20 W) in combination with blunt separation to minimize thermal injury of the urethra.
The UD was opened along the maximum axis to present its whole inner constitution (Figure 1C). At the moment, two small “S” shaped retractors would facilitate exposure. Careful exploration was performed, especially for multiloculated UDs. After 200 mL saline solution was injected into the bladder, one assistant pressed suprapubic region to increase inner-pressure of the bladder and urethra. The saline solution flowed towards the diverticular ostia through the urethra. The surgeon located the diverticular ostia from transvaginal perspective, where the saline solution flowed out (Figure 1C).
Electrocoagulation in low energy (20 W) and interrupted sutures with 4-0 Vicryl were performed to de-epithelialize and definitely close the ostia (Figure 1D). For large and multilocular UDs, the septa were bluntly separated to thoroughly expose the ostia. Redundant diverticular walls were removed for pathologic examination to exclude malignancy. Electrocoagulation in low energy (20 W) was used to de-epithelialize the remaining inner diverticular wall, which would be just large enough to cover the ostium in two layers.
Finally, the urethra would be closed and strengthened in three layers. First, interrupted sutures with 4-0 Vicryl were performed to close the remaining diverticular wall as imbrication (Figure 1E). The second step aimed to strengthen the urethra, especially for the weak part at 5 and 7 o’clock of the midpiece. The periurethral fibromuscular tissue was fixed on the repaired UD with 4-0 Vicryl in interrupted sutures. Third, the inverted U-shaped flap was stitched back with 4-0 Vicryl in continuous sutures (Figure 1F). The pattern diagrams of the surgical procedure were presented in Figure 2.
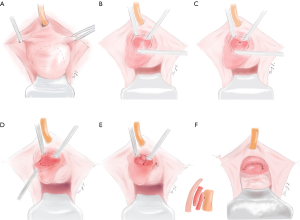
Postoperatively, all patients received intravenous antibiotics for 3–5 days. Vaginal povidone-iodine gauze was removed 24 hours after surgery. Vaginal irrigation with diluted povidone-iodine was performed on the postoperative 4th day. Urethral catheters were removed on postoperative 21 days. The patients were instructed to avoid intercourse for postoperative 3 months. In the postoperative first year, the patients were required to be re-evaluated every 3 months in terms of symptom alleviation, physical examination and transperineal ultrasound.
Results
Patient characteristics
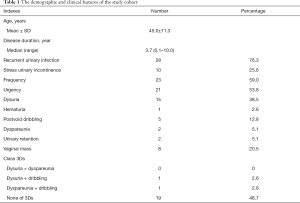
Overall, the present study included 39 patients with mean age of 45 (range: 24–78) years. The mean duration between initial symptoms and final diagnosis was 3.7 (range: 0.1–10) years. There were 28 patients (76.3%), 23 patients, and 21 patients (53.8%) suffering from recurrent urinary infection, urinary frequency and urgency, which were the most common symptoms of the study cohort (Table 1). Ten (25.6%) patients had stress urinary incontinence preoperatively. According to the classic 3Ds symptoms (dysuria, dyspareunia and dribbling), only 2 out of 39 patients had more than one symptom (dysuria and dribbling in one case; dyspareunia and dribbling in one case). As many as 19 out of 39 (48.7%) patients were free from any of classic 3Ds.
Full table
Characteristics of UDs
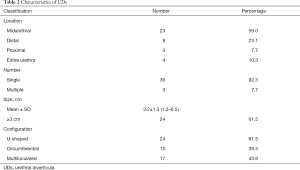
We diagnosed UDs mainly basing on cross-sectional postvoiding MRI. Meanwhile, the transperineal ultrasound was performed to record characteristics of UDs. The mean (SDs) size of UDs in the present study was 3.2 (1.3) cm (Table 2). There were 3 (7.7%), 9 (23.1%) and 23 (59.0%) patients having UDs located at proximal, midpiece and distal urethra. Another 4 (10.3%) patients had UDs extending whole length of the urethra. Multiple UDs were detected in 3 out of 39 patients. All of the 39 patients were classified as complex cases because of U-shaped diverticula (24/39) and circumferential diverticula (15/39). Multiloculated diverticula were detected in 17 out of 39 patients.
Full table
Surgical outcomes
Table 3 lists the surgical outcomes. The mean (SDs) operative time for the present surgical technique was 117.7 (34.7) minutes, and the mean postoperative hospitalization duration was 5.3±1.2 days. During the median follow-up time of 2.0 (range: 1.0–12.0) years, the number of cases with dyspareunia and LUTS were 4 and 3. There was no case of de novo urinary incontinence. However, two patients still had mild stress urinary incontinence during the follow-up, which were alleviated by biofeedback of pelvic movement or pelvic floor exercise. At postoperative 3 months, five patients had postoperative paraurethral cysts with the size ranging from 0.3 to 0.4 cm, which were absorbed in follow-up. Urinary retention occurred in 1 case, which was alleviated by prolonged catheterization until postoperative 3 months. For surgical pathology, no malignant lesion was reported, but one patient had atypical epithelium at diverticular wall.

Full table
Discussion
Management of symptomatic UDs remains a challenge in terms of non-specific symptoms and surgical modalities with minimal invasion. According to the published literatures, it is the storage LUTS but not traditional triad of symptoms (3Ds) that disturb most of the UD patients (2,3). In the present study, we made final diagnosis mainly basing on disease history, physical examination, transperineal ultrasound and MRI (14-16). Post-voiding MRI provides superb soft tissue contrast and is considered an ideal technique for delineating UDs. However, it is associated with higher cost and has some contraindications (5). In our center post-void MRI and transperineal ultrasound were performed for UD patients preoperatively. And ultrasound presentations correlated well with surgical findings, including location, configuration, septum, calcification, etc. (17,18). Meanwhile, transperineal ultrasound was also applied during follow-up. The repair of UDs began on cystourethroscopic examination (15). In this step, a rigid nephroscope with the water outlet at its front tip was used. Not only the location of a diverticular ostium on the urethra was recorded, but also the diverticulum was filled up to extend, which facilitated transvaginal diverticular detection in the following surgical procedure. In our experience, most of UDs locate at 5 and 7 o’clock of the mid-distal urethra, where distributes paraurethral glands.
Numerous surgical modalities of diverticulectomy to utmost restore urethral function have been reported, including total excision of the diverticular wall, the Spence procedure, Martius flap interposition, and so on (6,9-11,16-20). In Reeves et al. study, total dissection of the diverticulum was performed (9). And Martius flaps were interposed for cases with large defect (9). Nickles et al. conducted urethral transection and end-to-end anastomosis for the complex UDs (6). In our encountered series, the surgical technique had been implemented in all types of diverticula in terms of the location (proximal, midpiece or distal urethra), the number (single or multiple), and the configuration (U-shaped diverticula, circumferential diverticula or multiloculated diverticula). Our surgeons focused on definite closure of the ostia of UDs and strengthened urethral reconstruction. In our opinion, absolute closure of the communication between the urethral lumen and diverticular sac plus partial diverticulectomy not only enhanced curative rate but also decreased postoperative complications, for instance fistula, urinary incontinence, etc. (19). The diverticulum was opened along its maximum axis. After 200 mL saline solution was injected into bladder, one assistant pressed suprapubic region to increase inner-pressure of the bladder and urethra. The saline solution would flow towards the diverticular ostia through urethra. The surgeon would locate the diverticular ostia from transvaginal perspective, where the saline solution flowed out. Therefore, the communication between the diverticular lumen and the urethra could be definitely closed.
Surgical site infection is one of the most common early postoperative complications (9). In Reeves et al. study, one urinary tract infection and one Martius graft infection occur in 89 patients (9). In our series, all patients received intravenous antibiotics for 3 to 5 days. Vaginal povidone-iodine gauze was removed 24 hours after surgery. Vaginal irrigation with diluted povidone-iodine was performed on the postoperative 4th day. None postoperative infection was recorded. Meanwhile, in Reeves et al. study, three patients (3.4%) had recurrent residual UDs following surgery (9). In our study, at postoperative 3 months, five patients had paraurethral cysts with the size ranging from 0.3 to 0.4 cm. According to the transperineal ultrasound during the follow-up, the paraurethral cysts were effusion or hematoma between the sutured diverticular walls and the closed ostia. The effusion or hematoma were absorbed in follow-up. And no accompanying LUTS were recorded. According to the published literatures, the overall successful rate for transvaginal excision of UD is about 90% (18). Of note, absolute removal of diverticular walls was done in almost all of the previous studies (18). Therefore, the surgical method of partial excision of diverticular walls had a comparable cure rate with total excision of diverticular walls. In our mind, partial diverticulectomy not only minimized surgical trauma but also strengthened the urethra by remaining diverticular wall.
Stress incontinence often coexists in 10–57% women with UDs. And one of the most important indexes to evaluate surgical efficacy is postoperative continent function. Based on published studies, the occurrence rates of stress urinary incontinence after surgical treatment for UDs range from 1.7% to 16.1% (21). Although de novo stress urinary incontinence after the surgery is not rare, most of the cases belong to mild type (19). The rate of postoperative incontinence was 18.2% in urethral transection plus end-to-end anastomosis by Nickles et al. (6) and 14.6% in total dissection of UDs by Reeves et al. (9). In the present study, stress urinary incontinence was recorded in 10 (25.6%) patients preoperatively. However, 2 (5.1%) patients still had mild stress urinary incontinence during the follow-up, which were treated by biofeedback of pelvic movement or pelvic floor exercise. In the opinions of our surgeons, partial diverticulectomy preserved periurethral supported fibromuscular tissue, especially the sphincter complex for the UDs at proximal urethra and the UDs extending whole length of the urethra (19). Furtherly, the remaining diverticular walls, periurethral supported fibromuscular tissue, and the separated vaginal flaps made the strengthened urethral reconstruction. Meanwhile, the surgeons in our center suggested staged instead of simultaneous sling placement for stress urinary incontinence. First, more than half of preoperative urinary incontinence could be alleviated after the diverticulectomy plus urethral reconstruction. In Reeves et al. study, twenty out of 32 patients had urinary incontinence cured after UD excision (5). Meanwhile, partial diverticulectomy allows periurethral supported fibromuscular tissue to be preserved, which is essential to protect urethral continent function (19). Second, increasing risk of infection is another concern, especially when the urethra is entered during diverticulectomy.
The limitation of this study is its relatively small sample size and retrospective characteristics. Meanwhile, lack of comparison from a control group by traditional surgical method is another limitation.
Conclusions
Management of symptomatically complex UD is challenging. Transvaginal partial diverticulectomy, definite closure of diverticular ostia, and layered reconstruction of urethra have excellent urethral function protection and favorable surgical outcomes. Comparison of the present surgical technique to classic modalities is imperative to further evaluate its efficacy.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-478
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-478). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J 2014;25:73-9. [Crossref] [PubMed]
- Baradaran N, Chiles LR, Freilich DA, et al. Female urethral diverticula in the contemporary era: is the classic triad of the "3Ds" still relevant? Urology 2016;94:53-6. [Crossref] [PubMed]
- Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol 2000;164:428-33. [Crossref] [PubMed]
- Greenwell TJ, Spilotros M. Urethral diverticula in women. Nat Rev Urol 2015;12:671-80. [Crossref] [PubMed]
- Rovner ES. Bladder and Female Urethral Diverticula. In: Wein AJ, Kavoussi LR, Novick AC, et al. Campbell-Walsh Urology. 10th edition. Philadelphia: Saunders; 2011:2276.
- Nickles SW, Ikwuezunma G, MacLachlan L, et al. Simple vs complex urethral diverticulum: presentation and outcomes. Urology 2014;84:1516-9. [Crossref] [PubMed]
- Foley CL, Greenwell TJ, Gardiner RA. Urethral diverticula in females. BJU Int 2011;108 Suppl 2:20-3. [Crossref] [PubMed]
- Lee JW, Fynes MM. Female urethral diverticula. Best Pract Res Clin Obstet Gynaecol 2005;19:875-93. [Crossref] [PubMed]
- Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol 2014;66:164-72. [Crossref] [PubMed]
- Saito S. Usefulness of diagnosis by the urethroscopy under anesthesia and effect of transurethral electrocoagulation in symptomatic female urethral diverticula. J Endourol 2000;14:455-7. [Crossref] [PubMed]
- Roehrborn CG. Long term follow-up study of the marsupialization technique for urethral diverticula in women. Surg Gynecol Obstet 1988;167:191-6. [PubMed]
- Leach GE, Sirls LT, Ganabathi K, et al. L N S C3: a proposed classification system for female urethral diverticula. Neurourol Urodyn 1993;12:523-31. [Crossref] [PubMed]
- Rovner ES, Wein AJ. Diagnosis and reconstruction of the dorsal or circumferential urethral diverticulum. J Urol 2003;170:82-6; discussion 86. [Crossref] [PubMed]
- Seth JH, Naaseri S, Solomon E, et al. Correlation of MRI features of urethral diverticulum and pre- and post-operative stress urinary incontinence. Neurourol Urodyn 2019;38:180-6. [Crossref] [PubMed]
- Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg 2011;17:264-71. [Crossref] [PubMed]
- Kim B, Hricak H, Tanagho EA. Diagnosis of urethral diverticula in women: value of MR imaging. AJR Am J Roentgenol 1993;161:809-15. [Crossref] [PubMed]
- Wang X, Dou C, Yan Y, et al. Ultrasonographic features of female urethral diverticula: a retrospective study of 25 patients. Female Pelvic Med Reconstr Surg 2017;23:343-7. [Crossref] [PubMed]
- Wang X, Dou C, Yan Y, et al. Preoperative transurethral contrast-enhanced ultrasonography in the diagnosis of female urethral diverticula. J Ultrasound Med 2018;37:2881-9. [Crossref] [PubMed]
- Neveü P, Ouzaid I, Xylinas E, et al. Managing female urethral diverticulum with a standardized technique using a pacifier-trick artifice to facilitate dissection. Int Urogynecol J 2019;30:789-94. [Crossref] [PubMed]
- Crescenze IM, Goldman HB. Female urethral diverticulum: current diagnosis and management. Curr Urol Rep 2015;16:71. [Crossref] [PubMed]
- Lee UJ, Goldman H, Moore C, et al. Rate of de novo stress urinary incontinence after urethal diverticulum repair. Urology 2008;71:849-53. [Crossref] [PubMed]


