
Serum and tissue syndecan-1 levels in renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is one of the most common urologic cancers with an estimated incidence of 4.4/100,000 worldwide (1). The standard treatment of localized disease is surgery with the option of a nephron sparing approach, depending on tumor size and localization (2). In larger tumors or when the localization of the tumor is unfavorable, radical nephrectomy is recommended. Even large tumors involving the vascular system can be surgically removed providing beneficial impact for tumor related prognosis. In metastatic disease, multiple pharmacological treatment options are available. Even in metastatic disease, nephrectomy and metastatectomy in selected cases can be performed in order to improve of cancer-specific survival (CSS) (3,4).
Syndecan-1 (SDC1, CD138) is one out of four members of the transmembrane heparan sulfate proteoglycan family (5). In mammals SDC1 is mainly expressed in epithelial and mesenchymal cells. The transmembrane structure implicates its significance in cell-cell- and cell-microenvironment interactions. Syndecans are involved in non-malignant processes like wound healing, inflammation and neovascularization (6) as well as in the development and progression of numerous cancer types (7-10). Various growth factors like tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β) and basic fibroblast growth factor (bFGF) showed modulatory impact on SDC1 expression (11,12). SDC1 serves as co-receptor for various growth factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), bFGF and TGF-β (13,14). Substantial cell functions in the premalignant and malignant cell phenotype like altered migration, proliferation and cell motility, reduced adhesion and modified invasion capabilities were shown to be affected by SDC1 (7-10).
The extracellular domain of SDC1 can be shed resulting in a biological active ectodomain (15), which is able to trap the above-mentioned ligands acting as a competitive antagonist to the intact transmembrane protein, thus downregulating downstream receptor cascades (16).
In vitro studies demonstrated that reduced SDC1 expression levels are associated with altered cancer cell growth by modification of the microenvironment in a pro-malignant manner (17). It was shown that low SDC1 protein expression in tumor cells was associated with reduced prognosis and worse tumor related conditions in different solid tumor types including breast, head and neck, colorectal, bladder and prostate cancer as well as cholangiocarcinoma (18-20). On the other hand high SDC1 epithelial expression was associated with favorable outcome in squamous cell lung cancer (21). Furthermore, elevated serum concentration of shed SDC1 was associated with reduced survival in lung, bladder and prostate cancer (22,23).
Little is known about the role of SDC1 in RCC. Therefore, the aim of the present study was to assess the correlation of serum/tissue levels of SDC1 with clinicopathological parameters and follow-up data in RCC.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tau-19-787).
Methods
This retrospective study included 413 patients who underwent rule-based surgical therapy for RCC between 1990 and 2005. Preoperative serum samples were available for 100 patients, while formalin-fixed paraffin embedded (FFPE) tissue samples were available for 343 patients. In 52 cases, both FFPE tissue and serum samples were available. The study was performed according to the Declaration of Helsinki and the institutional ethics committee approved the study protocol (14-5738-BO). The primary endpoint of this study was overall survival (OS) and the secondary endpoints were CSS and recurrence free survival (RFS). All patients were followed from baseline (date of surgery) until December 2016. Clinical and pathological data was obtained from patients’ medical reports.
Syndecan serum expression, ELISA
Data on serum SDC1 (sSDC1) serum concentration was available from 100 patients. sSDC1 serum levels were quantified by using a sandwich ELISA (Diaclone CD138, Gene-Probe San Diego CA USA; Cat.Nr.: 950.640.096) according to the manufacturer’s instructions.
Histopathological work-up and immunohistochemistry (IHC)
Diagnosis was conducted in line with the WHO classification-scheme (24). FFPE tumor samples were available from 343 RCC patients. A tissue microarray (TMA) was constructed with three cores from each tumor sample after selection and labeling of the corresponding area on a hematoxylin & eosin slide.
Staining procedures for SDC1 were performed as described previously (25). Combined quantitative and qualitative evaluation of IHC results were performed by one pathologist blinded to clinical/follow-up data using a semiquantitative approach. Staining intensity was assessed as strong (3 points), moderate (2 points), weak (1 point) and no immunoreactivity (0 points) of all tumor cells.
Statistical analysis
Data are presented as medians ± SEM. Statistical significance was assigned at the level of P<0.05. Data lacking normal distribution were analyzed by the non-parametric two-tailed Wilcoxon rank sum test (Mann-Whitney) for paired group comparisons. Proportional distribution of the immunhistochemical results were analyzed using the Fisher’s exact test. OS, CSS and RFS analyses were done by uni- and multivariable Cox proportional hazard survival regression analyses and Kaplan-Meier survival analyses with log-rank (Mantel-Cox) test, using the IBM® SPSS® (version 24.0, Chicago, IL, USA) and GraphPad Prism® (version 6, La Jolla, CA, USA). As cut-off for serum expression, the median serum expression value was chosen. In the IHC results, strong and moderate signal was compared to weak and no signal. Perioperative variables with a P value of at least 0.05 in the univariable Cox-regression analyses were included in the multivariable models.
Results
Study population
Median patient’s age was 63 years (10–91 years), the male to female ratio was 2:1. Mean follow up time was 90.2 months. Median OS and RFS were 71.5 months (1–293 months) and 63.0 months (1–218 months). Five and 10 years OS were 67.1% and 56.0%, 5 and 10 years CSS were 78.5% and 75.8%. Data on local tumor stage was available from 307 patients (T1 38.4%, n=118, T2 23.8%, n=73, T3 36.2%, n=111 and T4 1.6%, n=5). Organ confined tumors (≤T2) were found in 62.2% whereas 37.8% were locally advanced (≥T3). Seven percent of the tumors were lymph node positive and in 9.8% metastatic disease was identified at the initial diagnosis.
Histopathological findings
Clear cell RCC was diagnosed in 78.7% of the tumors, papillary RCC in 16.4%, chromophobe RCC in 4.7% and sarcomatoid de-differentiated RCC in 0.5%. Distribution of Fuhrman nuclear grading 1–4 was 13.5%, 39.2%, 34.2% and 13.2%, respectively.
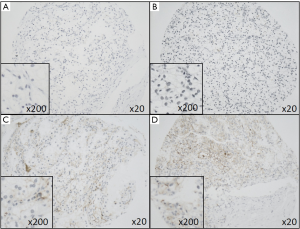
Beside membranous SDC1-reactivity in tumor cells, SDC1 (CD138) is typically positive in plasma cells and also in the plasma itself (Figure 1).
Correlation of sSDC1 concentration levels with clinicopathological parameters
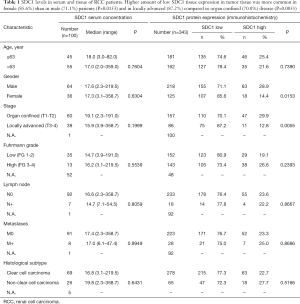
SDC1 serum concentration levels showed no correlation with patients’ age, gender, tumor stage, differentiation (Fuhrman nuclear grade), histological subtype, lymph node and/or metastasis status (Table 1). Median sSDC1 levels showed no difference between benign controls (11.0 ng/mL) and tumor patients (17.6 ng/mL; P=0.0769, n=100).
Full table
Correlation of tissue SDC1 expression with clinicopathological parameters
SDC1 protein expression showed no correlation with patients’ age, differentiation (Fuhrman nuclear grade), histological subtype, lymph node and/or metastasis status. In contrast, in female patients the content of low SDC1 protein expression was elevated compared to male patients (85.6% vs. 71.1%, P=0.0153, n=343). In addition, the content of low SDC1 protein tissue expression was elevated in locally advanced compared to organ confined disease (87.2% in ≥T3 vs. 70.0% in ≤T2, P=0.0055, n=243) (Table 1).
In case of 52 patients both, serum and tumor tissue samples were available. We found no correlation between sSDC1 serum levels and tissue immunoreactivity (P=0.7363).
Survival analysis
There was no difference in OS, CSS or RFS stratified by serum sSDC1 or SDC1 tissue protein levels (OS serum: P=0.1996; OS protein: P=0.8201; CSS serum: P=0.4900; CSS protein: P=0.2773; RFS serum: P=0.7837; RFS protein: P=0.1028) as shown in Kaplan-Meier survival analysis (Figure 2).

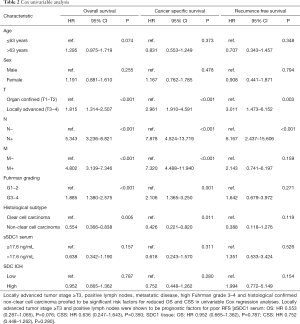
Univariable Cox regression analysis identified locally advanced tumor stage ≥T3, positive lymph node and distant metastasis status, Fuhrman grade 3–4 and histological subtype clear-cell carcinoma as significant risk factors for reduced OS (T, N, M, Fuhrman grading P<0.001, clear-cell carcinoma P=0.005) and CSS (T, N, M: P<0.001, Fuhrman grading P=0.001, clear-cell carcinoma P=0.011). Locally advanced disease ≥T3 and positive lymph nodes were identified as risk factors for reduced RFS (T: P=0.003, N: P<0.001) (Table 2) . sSDC1 levels and SDC1 protein tissue expression had no impact on OS or CSS in univariable analyses (sSDC1 serum: OS: P=0.157; CSS: 0.311; SDC1 protein: OS: P=0.787; CSS: P=0.280).
Full table
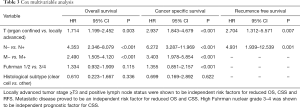
In the multivariable analyses locally advanced tumor stages ≥T3, positive lymph node and metastases status were shown to be independent risk factors for reduced OS (T: P=0.003, N and M: P<0.001) and CSS (T, N and M: P<0.001). Additionally, Fuhrman nuclear grading was shown to be an independent risk factor for reduced CSS (P<0.001). Locally advanced tumor stages ≥T3 and positive lymph node status proofed to be independent prognostic factors for reduced RFS (T: P=0.007, N: P=0.001) (Table 3).
Full table
Discussion
Dysregulation of SDC1 expression was frequently observed in various cancers. Both enhanced and decreased tissue expressions were found to be associated with clinicopathological parameters and patients’ prognosis in tissue-specific manner (17,18). For hematological tumors, SDC1 seems to have a significant clinical potential, as its enhanced serum levels were consistently shown to be associated with poor prognosis in chronic lymphocytic leukemia (26), large B-cell lymphoma (27), Hodgkin’s lymphoma (28) and multiple myeloma (29). Inconsistent results were shown for some solid tumors especially breast cancer, where dysregulation of SDC1 showed discrepant results. On the one hand elevated protein SDC1 expressions in immunhistochemical analyses showed reduced OS and CSS with reduced cancer related prognosis in advanced breast cancer (30-32). Other studies in contrast could not confirm this observation and showed no impact of SDC1 expression levels in ductal carcinoma of the breast (33) or adverse results with elevated numbers of high grade malignancies and reduced cancer related prognosis in invasive ductal breast cancer patients (34). A meta-analysis of ten studies with 888 colorectal carcinoma patients found decreased SDC1 expression levels in tumor compared to normal tissue as well as in low grade and low stage compared to high grade and high stage disease. However, pooled data analyses revealed no impact for SDC1 on patients’ survival (35).
In bladder cancer, decreased SDC1 expressions of tumor cells and increased SDC1 staining in tumor neighboring stromal cells were associated with progressed tumor stages and poor patients’ prognosis (36,37). Additionally, in our previous analysis, increased sSDC1 concentrations were associated with poor CSS in bladder cancer patients (36). In prostate cancer, SDC1 immunoreactivity showed no cancer related prognostic impact (23). Similar to bladder cancer, increased sSDC1 levels were found to be independently associated with poor CSS in advanced cases of prostate cancer, implicating the impact of shed SDC1-ectodomain as a preoperative risk-stratification parameter in prostate cancer (23). This thesis was underlined by the finding that SDC1 shedding seems to be involved in chemotherapy resistance in prostate cancer. In a cohort of 75 patients, increasing sSDC1 levels were associated with a declining therapeutic effect of docetaxel chemotherapy and worse cancer related prognosis (38).
SDC1 (also known as CD138) tissue expression is a well known marker for plasma cells in IHC. In a study with 50 RCC specimens (40 clear cell, 6 papillary and 4 chromophobe) SDC1 protein staining showed membranous immunoreactivity for clear-cell and chromophobe RCC whereas immunoreactivity was located at the basal cytoplasm in papillary RCC. Decreased SDC1 protein expression was associated with increasing nuclear grade. Correlations between SDC1 and tumor stage or histological subtypes could not be identified (39). In addition, our data on a larger number of RCC tumor samples showed a higher proportion of tumors with low SDC1 immunoreactivity in advanced stages of RCC. Interestingly, low SDC1 protein tissue expression was more often found in females compared to male patients.
None of the formerly published studies assessed the correlation between SDC1 and patients’ prognosis in RCC. Thus, to best of our knowledge, the present study is the first to assess this topic. Our data suggest that SDC1 tissue expression has no impact on OS, CSS or RFS in RCC. Similarly, analyzing the soluble SDC1 levels in preoperative serum samples of RCC patients for the first time, we found no correlation between SDC1 concentration and clinicopathological parameters or patients’ outcome. In our study, multivariable Cox survival analyses confirmed that clinical parameters including tumor stage, presence of lymph node or distant metastases as well as histopathological features such as histological subtype and Fuhrman nuclear grade are independent prognostic factors for RCC.
The present study has some potential limitations. First, the retrospective character of the study limits its informative value as all data of retrospective investigations should be interpreted critically. Further, a semiquantitative immunohistochemical approach is associated with increased susceptibility of systemic bias as the choice of antibody, staining method and subjective nature of the evaluator can influence the results.
Conclusions
SDC1 tissue protein expression was higher in organ confined disease compared to locally advanced RCC and in males compared to female patients. There was no difference in sSDC1 expression levels stratified to age, gender, local tumor stage, lymph node and metastases status, nuclear grade and histological subtype. No significant impact on patients’ prognosis could be identified for serum sSDC1 expression and SDC1 tissue protein expression in the present study. Locally advanced tumor stage and positive lymph node status were independent prognostic factors for reduced OS, CSS and RFS. Additionally, positive metastases status was an independent prognostic factor for reduced OS and CSS and elevated nuclear grade for CSS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tau-19-787
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-19-787
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-787). AE reports other from Consultant/Lecturer IPSEN Pharma, other from Lecturer IPSEN Pharma, other from Consultant MEDAC, other from Consultant Janssen CILAG, other from Lecturer Apogepha, outside the submitted work. ST reports personal fees and non-financial support from Ipsen, personal fees from Eisai, personal fees and non-financial support from Bayer, personal fees and non-financial support from Janssen, non-financial support from Novartis, non-financial support from BMS, non-financial support from BrachySolution, during the conduct of the study. BH reports grants, personal fees and non-financial support from Janssen, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Bayer, personal fees and non-financial support from Astellas, grants from German Cancer Aid, grants and other from Uromed, personal fees from Lightpoint Medical, personal fees from ABX, all outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this Original Article. The principles of the Helsinki Declaration were followed during the study. Patients were treated according to the Treatment Guidelines of the European Association of Urology and approved by an interdisciplinary tumor board. The Committee for Research of the Faculty of Medicine, University Hospital Essen, University Duisburg-Essen approved the study protocol (ID 14-5738-BO). Confidentiality of the patients’ data was guaranteed and all data was anonymized. Future management of the patients is not affected by the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Niedworok C, Dörrenhaus B, Vom Dorp F, et al. Renal cell carcinoma and tumour thrombus in the inferior vena cava: clinical outcome of 98 consecutive patients and the prognostic value of preoperative parameters. World J Urol 2015;33:1541-52. [Crossref] [PubMed]
- Bex A, Ljungberg B, van Poppel H, et al. European Association of Urology. The Role of Cytoreductive Nephrectomy: European Association of Urology Recommendations in 2016. Eur Urol 2016;70:901-5. [Crossref] [PubMed]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res 2005;96:488-500. [Crossref] [PubMed]
- Wu CY, Asano Y, Taniguchi T, et al. Serum level of circulating syndecan-1: A possible association with proliferative vasculopathy in systemic sclerosis. J Dermatol 2016;43:63-6. [Crossref] [PubMed]
- Lee H, Kim Y, Choi Y, et al. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Biophys Res Commun 2011;409:148-53. [Crossref] [PubMed]
- Lee JH, Park H, Chung H, et al. Syndecan-2 regulates the migratory potential of melanoma cells. J Biol Chem 2009;284:27167-75. [Crossref] [PubMed]
- Purushothaman A, Uyama T, Kobayashi F, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 2010;115:2449-57. [Crossref] [PubMed]
- Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol 2012;31:3-16. [Crossref] [PubMed]
- Cizmeci-Smith G, Langan E, Youkey J, et al. Syndecan-4 is a primary-response gene induced by basic fibroblast growth factor and arterial injury in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1997;17:172-80. [Crossref] [PubMed]
- Halden Y, Rek A, Atzenhofer W, et al. Interleukin-8 binds to syndecan-2 on human endothelial cells. Biochem J 2004;377:533-8. [Crossref] [PubMed]
- Salmivirta M, Heino J, Jalkanen M. Basic fibroblast growth factor-syndecan complex at cell surface or immobilized to matrix promotes cell growth. J Biol Chem 1992;267:17606-10. [PubMed]
- Chu CL, Buczek-Thomas JA, Nugent MA. Heparan sulphate proteoglycans modulate fibroblast growth factor-2 binding through a lipid raft-mediated mechanism. Biochem J 2004;379:331-41. [Crossref] [PubMed]
- Bass MD, Morgan MR, Humphries MJ. Syndecans shed their reputation as inert molecules. Sci Signal 2009;2:pe18. [Crossref] [PubMed]
- Nikolova V, Koo CY, Ibrahim SA, et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis 2009;30:397-407. [Crossref] [PubMed]
- Yang Y, Macleod V, Miao HQ, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem 2007;282:13326-33. [Crossref] [PubMed]
- Harada K, Masuda S, Hirano M, et al. Reduced expression of syndecan-1 correlates with histologic dedifferentiation, lymph node metastasis, and poor prognosis in intrahepatic cholangiocarcinoma. Hum Pathol 2003;34:857-63. [Crossref] [PubMed]
- Anttonen A, Kajanti M, Heikkilä P, et al. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br J Cancer 1999;79:558-64. [Crossref] [PubMed]
- Lundin M, Nordling S, Lundin J, et al. Epithelial syndecan-1 expression is associated with stage and grade in colorectal cancer. Oncology 2005;68:306-13. [Crossref] [PubMed]
- Anttonen A, Heikkilä P, Kajanti M, et al. High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer 2001;32:297-305. [Crossref] [PubMed]
- Joensuu H, Anttonen A, Eriksson M, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res 2002;62:5210-7. [PubMed]
- Szarvas T, Reis H, Vom Dorp F, et al. Soluble syndecan-1 (SDC1) serum level as an independent pre-operative predictor of cancer-specific survival in prostate cancer. Prostate 2016;76:977-85. [Crossref] [PubMed]
- Eble JN, Sauter G, Epstein JI. Pathology and Genetics. Tumours of the Urinary System and Male Genital Organs (WHO Classification of Tumours, Volume 7, Third Edition) WHO 2004, IARC, Lyon.
- Bayer-Garner IB, Nickell JA, Korourian S. Routine syndecan-1 immunohistochemistry aids in the diagnosis of chronic endometritis. Arch Pathol Lab Med 2004;128:1000-3. [PubMed]
- Jilani I, Wei C, Bekele BN, et al. Soluble syndecan-1 (sCD138) as a prognostic factor independent of mutation status in patients with chronic lymphocytic leukemia. Int J Lab Hematol 2009;31:97-105. [Crossref] [PubMed]
- Bodoor K, Matalka I, Hayajneh R, et al. Evaluation of BCL-6, CD10, CD138 and MUM-1 expression in diffuse large B-cell lymphoma patients: CD138 is a marker of poor prognosis. Asian Pac J Cancer Prev 2012;13:3037-46. [Crossref] [PubMed]
- Vassilakopoulos TP, Kyrtsonis MC, Papadogiannis A, et al. Serum levels of soluble syndecan-1 in Hodgkin's lymphoma. Anticancer Res 2005;25:4743-6. [PubMed]
- Andersen NF, Standal T, Nielsen JL, et al. Syndecan-1 and angiogenic cytokines in multiple myeloma: correlation with bone marrow angiogenesis and survival. Br J Haematol 2005;128:210-7. [Crossref] [PubMed]
- Nguyen TL, Grizzle WE, Zhang K, et al. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am J Clin Pathol 2013;140:468-74. [Crossref] [PubMed]
- Leivonen M, Lundin J, Nordling S, et al. Prognostic value of syndecan-1 expression in breast cancer. Oncology 2004;67:11-8. [Crossref] [PubMed]
- Tsanou E, Ioachim E, Briasoulis E, et al. Clinicopathological study of the expression of syndecan-1 in invasive breast carcinomas. correlation with extracellular matrix components. J Exp Clin Cancer Res 2004;23:641-50. [PubMed]
- Tiemann K, Weigel MT, Alkatout I, et al. Significance of syndecan-1 expression in ductal carcinoma in situ of the breast. Anticancer Res 2014;34:3607-16. [PubMed]
- Loussouarn D, Campion L, Sagan C, et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br J Cancer 2008;98:1993-8. [Crossref] [PubMed]
- Wei HT, Guo EN, Dong BG, et al. Prognostic and clinical significance of syndecan-1 in colorectal cancer: a meta-analysis. BMC Gastroenterol 2015;15:152. [Crossref] [PubMed]
- Szarvas T, Reis H, Kramer G, et al. Enhanced stromal syndecan-1 expression is an independent risk factor for poor survival in bladder cancer. Hum Pathol 2014;45:674-82. [Crossref] [PubMed]
- Kim JH, Park J. Prognostic significance of heme oxygenase-1, S100 calcium-binding protein A4, and syndecan-1 expression in primary non-muscle-invasive bladder cancer. Hum Pathol 2014;45:1830-8. [Crossref] [PubMed]
- Szarvas T, Sevcenco S, Módos O, et al. Circulating syndecan-1 is associated with chemotherapy-resistance in castration-resistant prostate cancer. Urol Oncol 2018;36:312.e9-312.e15. [Crossref] [PubMed]
- Gökden N, Greene GF, Bayer-Garner IB, et al. Expression of CD138 (Syndecan-1) in renal cell carcinoma is reduced with increasing nuclear grade. Appl Immunohistochem Mol Morphol 2006;14:173-7. [Crossref] [PubMed]


