
Computerized tomography before the final treatment cycle of neoadjuvant chemotherapy or induction chemotherapy in muscle-invasive urinary bladder cancer, cannot predict pathoanatomical outcomes and does not reflect prognosis—results of a single centre retrospective prognostic study
Introduction
In the Western world, urinary bladder cancer (UBC) is the 4th most common malignancy in men and 8th most common in women (1). While most newly diagnosed patients have non-muscle-invasive bladder cancer, urothelial muscle-invasive bladder cancer (MIBC) accounts for approximately 25% of new cases, with a five-year overall survival (OS) of approximately 50% in stages cT2-T4 after radical cystectomy (2). MIBC is associated with high risk of regional and distant metastatic spread, the latter with a median survival of 15 months albeit maximum oncological treatment (3). The rationale for Cisplatin-based neoadjuvant combination chemotherapy (NAC) is to eradicate micrometastatic disease at the best point of time, to be followed by radical cystectomy (RC) with regional lymph node dissection (2,4,5).
NAC has been shown to significantly increase OS for a chemo-sensitive sub-group of these patients with an absolute risk reduction (ARR) of 31% in completely downstaged patients (pT0N0M0) at five years median observation time (6). In a meta-analysis investigating 886 patients from 13 trials, the correlation between complete response (CR i.e., pT0N0M0) and improved OS has been reconfirmed (7).
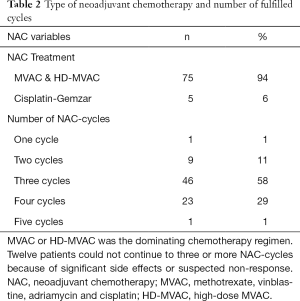
Standard treatment in Sweden, is three, and in some national centers four cycles of NAC, followed by RC, four to five weeks after final chemotherapy cycle (8).
In many centers in Sweden, including Norrland University hospital, Umeå (NUS), there is a routine of performing a control computerized tomography (cCT) prior to the final NAC-cycle. The same routine is used for patients undergoing induction chemotherapy (IC); namely patients with urothelial MIBC and minimal nodal dissemination (cN1-2) yet fit for both preoperative chemotherapy as well as RC. The claimed rationale for the approach of cCT, is to evaluate if the primary tumor is showing response to treatment or to identify regional or general progression of the cancer, comparing with baseline CT images obtained before start of NAC. In case of local progression, the ad hoc recommendation is to proceed quickly to RC without performing the final cycle of NAC/IC. In cases of very advanced progression or newly discovered disseminated disease—mainly for NAC-patients, the local algorithm is to consider abortion of RC and instead proceed to oncological treatment options. There is one single retrospective study supporting this routine, in which the investigators evaluated 59 patients undergoing NAC for MIBC. The study concluded that it was possible to evaluate the radiological response rate by pretreatment and post-chemotherapy CT, and that it was feasible to predict both the pathological outcome as well as the post-RC survival prognosis (9).
In the Swedish Northern health region (one of six national health regions), all newly diagnosed MIBC-patients are discussed regularly on a weekly basis, at the regional multidisciplinary team (MDT) meetings. For NAC-patients and fit patients with minimal nodal disease (pN1-2), the MDT-meetings take place in at least two occasions; primarily after conclusive histopathology following transurethral resection of the bladder tumour (TURb) and baseline CTs have been performed (CT urography and CT Thorax) and later on, following the second of three planned NAC-cycles (in some patients following the third of four planned NAC-cycles), the cCTs are performed and discussed. The standard MDT includes participation of urologists from the whole health region over audio-visual link, oncologists, radiologists, pathologists and specialized nurses. Organized and regular MDT-meetings are considered being highly recommendable for both increasing the usage of NAC and IC, as well as for improving options for diversified treatment selections for all MIBC-patients (10). Apart from the reported single center experience, the lack of solid evidence for the practice of performing a cCT before final NAC cycle, has specifically been addressed by the European Association of Urology and its guidelines-group on Muscle-invasive and Metastatic Bladder Cancer. A variety of imaging modalities (PET, CT, conventional MRI or DCE MRI) are clearly mentioned in their respective incapacity to accurately predict NAC-responses (2,9). The primary aim of the study was to analyze the ability of cCT, before the final chemotherapy cycle in mainly NAC patients, to predict histopathological pTNM-staging in terms of response and non-response to NAC—that concept considered being an established surrogate marker for prognostication in NAC patients (6,11). Secondary aims were to evaluate the ability of cCT to predict two- and three-year overall survival. We present this article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-19-872).
Methods
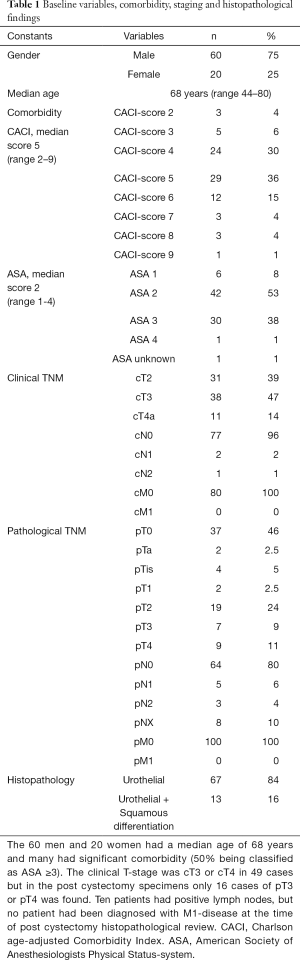
Inclusion criteria for this retrospective study, were patients who were diagnosed with urothelial muscle-invasive bladder cancer with or without squamous epithelial differentiation from the internal department-list of all patients who had undergone NAC and RC between the years 2006–2014 at a tertiary referral center - Norrland university hospital (NUS) in Umeå, Sweden. Seventy-seven NAC-patients with localized urothelial MIBC (cT2-4aN0M0) were identified, plus three patients with minimal nodal disease (cN1-2). The study database included patient data from all available patient records, as well as radiological and perioperative data from necessary documentation systems used at NUS. The included clinical data were; age, gender, clinical and pathological TNM staging, pre-RC CACI (Charlson age-adjusted Comorbidity Index), pre-RC ASA (Table 1) and specifications on NAC treatment (type, amount of cycles) (Table 2). For cTNM-staging, patients with uni- or bilateral hydronephrosis were classified as cT3. Baseline CTs (CT urography and CT thorax) were performed in conjunction with diagnostic TURb, to determine cN-status and cM-status. Patients with suspicious cM+ status, were not included in the study. For describing the whole RC-series of MIBC-patients receiving pre-RC chemotherapy the given years at our center, we also decided to include the three patients who received induction chemotherapy (two patients with cN1-disease and one staged preoperatively as cN2). Induction chemotherapy (IC) is a treatment strategy which is not defined as bona fide NAC-treatment, but for the completeness of the series these patients were also evaluated (2). For pTNM-staging post-RC we set following designations; pT0N0M0 for complete response (CR), pTaN0M0, pTisN0M0 or pT1N0M0 for partial response (PR), pT2-T4aN0M0 for stable disease (SD), pN+ and/or pM+ pT4b and/or tumour positive resection margin for progressive disease (PD).
Full table
Full table
RECIST criteria
The radiologist in the research group (EE), reviewed all patients base line CTs and the CTs performed prior to final chemotherapy cycle, with the intentions to classify the evaluations according to RECIST-criteria (Response Evaluation Criteria In Solid Tumors) into following categories; complete response (CR), stable disease (SD) or progressive disease (PD). For not mixing these evaluations with the similar set of response designations for post-RC histopathological evaluations, we designated CT-evaluations of complete response with CR-CT, stable disease with SD-CT, and progressive disease with PD-CT. Further our radiologist had to expand the number of radiological groups, to also include two new designations; mixed response (MIXRESP-CT), and difficult to interpret (DIFINTRP-CT) (Table 3).

Full table
Statistics
Standard descriptive statistics were used to display the findings in the results-section. First, we evaluated the results of the cCT scans in relation to the pathoanatomical outcomes post-RC. The index tests which were used, were the results of the cCTs performed prior to the final chemotherapy cycle. As the reference test for pathoanatomical outcomes, pTNM staging was used. The analysis of the primary outcome measurement included the 56 patients that could be dichotomized into either complete response (CR-CT) or no response; i.e., stable disease plus progressive disease (SD-CT + PD-CT) who had undergone complete assessments of both pathology post-RC as well as computed tomography. The main cohort of 80 patients was then analyzed in terms of OS at two- and further at three-year median time of observation, based on the different subgroups of pTNM. From the 80 patients, the 71 patients with complete radiological data, also including those with mixed response (MIXRESP-CT) and those who had scans difficult to interpret (DIFINTRP-CT) were then analyzed for OS at two- and for three-years median time of observation, based on the different subgroups of radiological staging (Figure 1). The same analysis was conducted using the pTNM-groups. A logistic regression was performed using the pTNM-groups and cCT-groups to analyze the two and three-year OS, since the outcome death, prior to two or three years, were expressed as binary events 1 or 0. The analyses were adjusted for age, gender and comorbidity (CACI). The data was analysed with IBM SPSS statistics 24, where we set a P value <0.05 as the indicator of statistical significance.
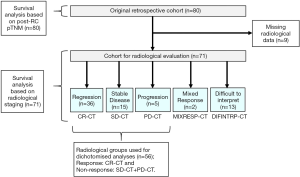
Results
The cohort had a female-to-male ratio of 1:3, a median age of 68 at RC, a median comorbidity score (CACI) of 5 and a median ASA score of 2. The pathological TNM-staging revealed that 46% of the patients were completely down staged to pT0, 10% had positive lymph nodes but none had been diagnosed with distant metastases (Table 1). Most patients had received MVAC or HD-MVAC (94%) and 87% had undergone three or four cycles of preoperative chemotherapy (NAC or IC) (Table 2). The results of the CT-analysis are shown in Table 3. Complete response (CR-CT) was found in 45% of the patients while 25% of the patients were considered to be non-responders (stable disease; SD-CT plus progressive disease PD-CT). Almost 20% of the patients had mixed response (MIXRESP-CT) or scans that were difficult to interpret (DIFINTRP-CT). The 56 patients who had undergone complete assessments of both pathology post-RC as well as cCT, with results that could be dichotomized into either complete response (CR-CT) or no response (SD-CT+PD-CT), were evaluated in comparison with the corresponding pTNM-staging of the post-RC specimens. The sensitivity of CT to predict non-responders according to pTNM was 64% (95% CI: 44–81%), the specificity was 36% (95% CI: 19–56%) the positive likelihood ratio was 1 (95% CI: 0.7–1.5) and the negative likelihood ratio was 1 (95% CI: 0.5–2). The predictive results were evenly distributed (fifty/fifty) and thus of no avail for accurate prediction (Table 4). When evaluating the 71 patient that also included those with MIXRESP-CT and those who were deemed DIFINTRP-CT we found that only 14/36 patients (39%) with CT-predicted regression had complete response (CR) and as many as six of 36 patients (17%) had progressed (PD). Notably four of five patients (80%), with computer tomography evaluations of PD (PD-CT), actually were CR; thus, only one of five (20%) had been correctly predicted with progressive disease (Table 5).

Full table

Full table
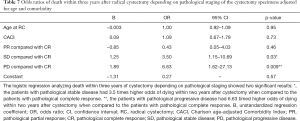
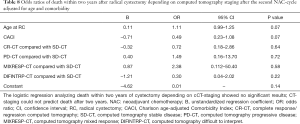
The odds ratio for two-year OS was calculated with pathological CR (pT0N0M0) as reference. A statistically significant increase in death, was seen in the pathological SD and pathological PD patient groups compared to CR patients (P=0.003 and P=0.005 respectively). The same correlation could not be seen when analyzing survival for PR patients to CR (P=0.86). The patients with progressive disease had 8.96 timed higher odds of dying within two years after cystectomy when compared to the patients with complete response (Table 6). Similar results were found when analyzing three-year survival in the same groups with a statistically significant increase in death for patients in the pathological SD and PD patient groups compared to CR (P=0.03 and resp. P=0.009) (Table 7).

Full table
Full table
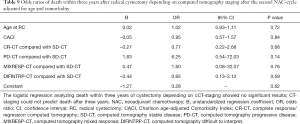
No statistically significant results were observed when analyzing two-year OS in comparing the radiological responses of the different groups (CR-CT, PD-CT, MIXRESP-CT and resp. DIFINTRP-CT) with the group of patients that radiologically were deemed to have a stable disease (SD-CT), in comparison with their respective baseline CT (Table 8). Analysis of the three-year OS in the same groups gave similar results as the two-year OS analysis (Table 9). Thus, the radiological response measured in CT-evaluations did not correlate to two- or three-year survival with any statistical significance.
Full table
Full table
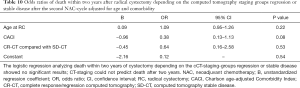
We also analyzed two and three-year OS, only considering the patients with complete response (CR-CT) compared to the group with radiological stable disease (SD-CT), without identifying any statistically significant results (Tables 10,11).
Full table

Full table
Discussion/Conclusions
The introduction of NAC plus RC, as a recommendation of treatment for all medically fit patients with urothelial MIBC, is based on a set of well performed randomized prospective trials displaying survival benefits (OS) ranging from 5–8% at five years of median observation time (2,4,5). Yet, as it is well known in analyzing both the mentioned trials as well as several register-based investigations, only 25–40% of treated patients show CR or PR—these pathoanatomical outcomes in their respective capacities of being surrogate markers of treatment efficacy in terms of longtime survival benefits (6,11,12). Thus, most of the patients will either have SD or PD even after three cycles of NAC and will consequently have received treatment leaving no impact on survival improvement. The delays for radical treatment (mainly RC) in that large subset of patients, are mainly ranging from 2.5 to four months, depending on specific chemotherapy regimen and local tradition—a delay that might hypothetically also have negative impact on the survival projections. Yet, there are yet no studies showing any negative survival impact, due specifically to the delays of NAC treatment. Extended lead times until RC as a negative factor for final pTNM-stages and with negative impact on long term survival, has only been evaluated in NAC-naïve MIBC-patients (i.e., undergoing RC only) (13-15). Further, until now there are no randomized prospective trials for MIBC-patients, evaluating IC plus RC versus chemonaïve treatment (RC only), in terms of survival benefits or pathoanatomical outcomes.
Our study was made to evaluate a routine using a control CT to determine if the patient is responding to neoadjuvant chemotherapy in urothelial MIBC or not. If deemed non-responsive with radiological signs of progression of cancer, the last cycle of chemotherapy would not be administered and the patient’s planned RC would be performed as quickly as possible, alternatively that the patient would be offered a non-surgical oncological option. We compared the outcomes and survival results of the surrogate markers of downstaging in the final cystectomy specimens (urinary bladder tumor with excised regional lymph nodes), to a parallel survival analysis guided by the results of resp. CT-scans performed prior to final cycle of NAC. As expected, our study displayed an improved overall survival when having a completely histopathologically downstaged cancer (pT0N0M0) at time of cystectomy versus still having a muscle invasive or an upstaged cancer in the main cohort of patients (n=80). Further, we showed that cCT had a poor correlation with the post-RC pTNM and could not predict OS. In our cohort of CT-evaluated patients (n=71), the patients were placed in groups according to the assessment of their radiological response after their cCT, the diversity of radiologic assessment-groups we had to include, shows an uncertainty using CT as a control method. Instead of only having three groups; regression (CR-CT), stable disease (SD-CT), and progression (PD-CT), our radiologist had to expand the number of groups to also include mixed response (MIXRESP-CT), and difficult to interpret (DIFINTRP-CT). The lack of predictive value for the control CT in relation to pathoanatomical response and non-response parameters in the post-RC specimens, was obvious. One major argument for using the control CT, is that it at least can inform the concerned clinicians about progressing patients (PD) and that the question of stable disease or not, is to be considered of less importance.
Yet, 80% with computer tomography evaluations of PD (PD-CT) had actually CR and only 20% had been correctly predicted with progressive disease (Table 5)—this deeming the control CT in terms of PD-prediction, rather useless. Thus, our findings further strengthen the mentioned negative recommendation from the EAU-Guidelines group, regarding the utilization of control CT in clinical practice (2).
Further, we found, when using cCT in routine manner, that there was lack of statistically significant results when comparing OS to CT results. We conclude that the cCT before final NAC cycle, did not contribute to any prognostic information, in our analysis. Yet the study was limited due to its retrospective nature and due to the moderate size of the cohort. A larger cohort would make it possible to adjust results with additional confounders. With a larger cohort, it would also be of interest to separately analyze true NAC-patients separately from patients who had undergone IC. Yet, there are at present no described radiological standards for that kind of comparative evaluations. The study covers 100% of all cystectomized patients fitting to the inclusion criteria at a high output urological department during a given time-period, and in contrast to the Swedish national cystectomy register, we have substantial amounts of detailed data on every included patient plus that we have validated all data; patient by patient (8). Larger retrospective trials and prospective trials are warranted.
In conclusion, control CT prior to the planned final preoperative chemotherapy-cycle in MIBC patients undergoing NAC or induction chemotherapy has a poor correlation with post-RC pTNM and cannot predict overall survival.
Acknowledgments
Funding: This work was supported by the Swedish Research Council funding for clinical research in medicine (ALF) in Västerbotten, VLL, Sweden and the Cancer Research Foundation in Norrland, Umeå, Sweden. We also thank assoc. Professor Johan Svensson, Umeå School of Business, Economics and Statistics (USBE), Umeå university, Sweden, for valuable support in analysis of the statistics.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-19-872
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-19-872
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-872). The authors have no conflicts of interest to declare. None of the authors have at any time received payment or services from a third party for any aspect of the submitted work. None of the authors have any patents; planned, pending or issued, broadly, relevant to the work. The four authors have no other relationships or activities that readers could perceive to have influenced or have given the appearance of potentially influencing the contents in article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Regional Ethics Board in Umeå: EPN-Umeå, dnr; 2013/463-31M with the amendment dnr; 2016/403-32. The study conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). The Regional Ethics Board had specifically decided that informed consent from the participants was to be considered redundant, especially due to the high mortality in MIBC as well as due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kirkali Z, Chan T. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66:4-34. [Crossref] [PubMed]
- Witjes JA, Compérat E, Cowan NC, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068-77. [Crossref] [PubMed]
- Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol 2004;45:297-303. [Crossref] [PubMed]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202-5; discussion 205-6. [Crossref] [PubMed]
- Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol 2012;61:1229-38. [Crossref] [PubMed]
- Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014;65:350-7. [Crossref] [PubMed]
- Jerlström T, Chen R, Liedberg F, et al. No increased risk of short-term complications after radical cystectomy for muscle-invasive bladder cancer among patients treated with preoperative chemotherapy: a nation-wide register-based study. World J Urol 2020;38:381-8. [Crossref] [PubMed]
- Fukui T, Matsui Y, Umeoka S, et al. Predictive value of radiological response rate for pathological response to neoadjuvant chemotherapy and post-cystectomy survival of bladder urothelial cancer. Jpn J Clin Oncol 2016;46:560-7. [Crossref] [PubMed]
- Sherif A. The long perspective in emergence of neoadjuvant chemotherapy for bladder cancer in Ontario, Canada-space for improvement with regular and organized multidisciplinary team meetings. Transl Androl Urol 2018;7:508-10. [Crossref] [PubMed]
- Lavery HJ, Stensland KD, Niegisch G, et al. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: a useful surrogate. J Urol 2014;191:898-906. [Crossref] [PubMed]
- Zargar H, Zargar-Shoshtari K, Lotan Y, et al. Final Pathological Stage after Neoadjuvant Chemotherapy and Radical Cystectomy for Bladder Cancer-Does pT0 Predict Better Survival than pTa/Tis/T1? J Urol 2016;195:886-93. [Crossref] [PubMed]
- Sánchez-Ortiz RF, Huang WC, Mick R, et al. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol 2003;169:110-5; discussion 115. [Crossref] [PubMed]
- Chang SS, Hassan JM, Cookson MS, et al. Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol 2003;170:1085-7. [Crossref] [PubMed]
- May M, Nitzke T, Helke C, et al. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol 2004;38:231-5. [Crossref] [PubMed]


