Deep vein thrombosis in a nonobstructive azoospermia male taking tamoxifen: a rare case report
Introduction
Tamoxifen is a selective estrogen receptor modulator, which is used for idiopathic male infertility for many years (1). About 10% of infertile men have azoospermia, which is divided into obstructive azoospermia (OA) and nonobstructive azoospermia (NOA). NOA is characterized by the complete absence of sperm in the ejaculate due to testicular failure. With the progress in assisted reproductive techniques (ART), spermatozoa could be surgically retrieved from these NOA patients, and provided for performing intracytoplasmic sperm injection (ICSI). However, in many cases of NOA, we are unable to find spermatozoa for ICSI. It is reported that tamoxifen can improve the results of sperm acquisition and the chance of pregnancy by microinjection.
Nonetheless, tamoxifen may increase the risk of venous thromboembolism (VTE), especially deep venous thrombosis (DVT), with a relative risk of 2- to 7-fold in the treatment of breast cancer (2). Unlike the mechanisms of estrogen, tamoxifen increases the level of procoagulant factors (factors VIII, IX, vWf), decreases the anticoagulant factors (antithrombin, total protein S, protein C, plasminogen activator inhibitor-1) (3).
However, the thromboembolic risk of tamoxifen in the treatment of male infertility is rarely reported. Here we present a rare case of a non-obstructive azoospermia patient who developed deep vein thrombosis (DVT) with the use of tamoxifen, probably through a hypercoagulable state. We present the following article in accordance with the CARE Guideline (4).
Case presentation
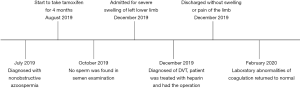
A 32-year-old Chinese man was admitted at the local hospital for severe swelling of left lower limb, with difficulty walking. The patient was alert, fully oriented, and had no fever or signs of infection. Five months ago, this patient had been diagnosed with NOA, and taken tamoxifen 20 mg daily for 4 months continuously. After the tamoxifen treatment 2 months, no sperm was found in semen examination, without blood test. Moreover, He had a normal medical examination last year, and normal body mass index (BMI) 19.8 kg/m2, without obvious family history and other medical history.
Upon admission, a lower limbs Doppler ultrasound of the deep venous system was performed, showing an absolutely obstruction with internal thrombus from the left femoral vein to the left iliac vein. Then, blood tests were performed before anticoagulant therapy. D-dimer was elevated to 12.61 mg/L (normal range of 0–0.5). The rest of the blood coagulation indicators were normal, including prothrombin times, prothrombin activity, international normalized ratio, activated partial thromboplastin time, fibrinogen concentration and thrombin time. While the hemoglobin and red blood cells were decreased to 124 g/L (normal range of 130–175) and 4.16×1012/L (normal range of 4.3×1012–8×1012). The platelet count was within the normal range.
After the diagnosed of DVT, he was treated with anticoagulant-low molecular weight heparin (LMWH) 12,000 IU daily for 2 days. On the second day of admission, he underwent local anesthesia for the surgery of percutaneous venous thrombus aspiration, iliac vein balloon dilatation, and implanted inferior vena cava filter extraction. He was discharged home three days after surgery without swelling or pain of left lower limb.
After discharged, the patient took rivaroxaban 20 mg daily for 2 months. And the subsequent laboratory abnormalities of coagulation returned to normal. Additionally, he didn’t feel any discomfort. The timeline picture was shown in Figure 1.
Discussion
NOA is the most severe form of male infertility, which comprises for about 60% of azoospermia cases. According to the pathogen, NOA can be divided into three types: congenital NOA, acquired NOA, and idiopathic NOA. At present, most patients need to try surgical sperm extraction for ICSI treatment, except for some patients with definite etiology and a few idiopathic NOA who can obtain ejaculatory sperm through treatment (5).
Spermatogenesis is a complex process that requires the complete hypothalamic-pituitary-gonadal axis. Hormone therapy for male infertility is a neglected and underutilized treatment which can have a significant impact on the clinical outcomes (6). Tamoxifen citrate is a nonsteroidal selective anti-oestrogen receptor modulator commonly used for the treatment of idiopathic male infertility. It increases pituitary secretion by blocking feedback inhibition of oestradiol, thus increasing endogenous GnRH secretion from the hypothalamus, and FSH and LH secretion directly from the pituitary gland. FSH can promote spermatogenesis directly. LH stimulates Leydig cells to produce testosterone, thereby boosting spermatogenesis with a possible improvement in fertility. A meta-analysis demonstrated tamoxifen as empiric medical therapy for idiopathic male infertility can improve sperm concentration and per cent sperm motility, increase spontaneous pregnancy rate (7). Used for NOA, tamoxifen can improve the results of sperm recovery in testis samples and increase the chance of pregnancy by microinjection (8).
Despite these benefits, the use of tamoxifen has raised concerns about the increased risk of thrombosis complications (9). In a recent case-control study, tamoxifen use is associated with 1.95-fold increased odds of deep vein thrombosis or pulmonary embolism among older women with breast cancer (10). Furthermore, tamoxifen use was associated with at least 41% higher VTE risk compare with aromatase inhibitor (11). Nevertheless, thrombosis complication of tamoxifen mostly focused on the treatment of breast cancer. There was rare literature about the VTE complication of tamoxifen treatment on male infertility, especially NOA. Exclusively, Allasia et al. reported a case of idiopathic male infertility who developed DVT with the use of tamoxifen for 3 months (12). Considering the hemostatic system in cancer patients is influenced by confounding factors such as the malignant disease itself and its treatment by surgery, chemotherapy, and radiotherapy, it can better clarify the effects of tamoxifen on coagulation function under the use in NOA patient, who has no other diseases.
The mechanisms by which tamoxifen increases the risk of thrombosis is not yet fully elucidated, although a decrease of several inhibitors of coagulation or an APC resistance phenotype has been reported (13-15). This patient had no previous history of diseases related to thrombosis. Hence, we concluded the main cause of the DVT was related to tamoxifen.
The diagnostic method of DVT mainly includes clinical symptom assessment, d-dimer detection and Doppler ultrasonography (16). Anticoagulant therapy can effectively reduce the risk of thrombosis and the probability of thrombosis recurrence, which is the basis of acute DVT therapy. Anticoagulant therapy should be performed when the diagnosis is established or highly suspicious. Common anticoagulant drugs involve heparin, LMWH, Vitamin K inhibitor, and so on. For patients with acute iliofemoral DVT and without cancer, treatment with the regimens may be initiated: low-molecular weight heparin, with switch after 1 week to rivaroxaban (17). Mechanical thrombus aspiration can reduce thrombotic complications, including a significant reduction of the incidence of post-thrombotic syndrome, safer than surgical thrombectomy and systemic thrombolysis (18). Therefore, this patient underwent the surgery of venous thrombus aspiration, received LMWH and rivaroxaban successively. Finally, he was satisfied with the therapeutic efficacy.
To our knowledge, this is the second published case of thrombosis in male infertility after treated with tamoxifen, but the first case of NOA patient. And this patient has no other diseases, which can well explain the correlation between tamoxifen and DVT. However, this case reported has some limitations. Firstly, we reported just one case, because of the low incidence clinically. Secondly, limited to local conditions, the patient did not receive more tests for coagulation related factors, such as factors VIII, protein C, and protein S, that may better elucidate the mechanism of tamoxifen related to thrombosis.
Conclusions
The DVT occurred in this case was probably caused by the use of tamoxifen. Although a single case is insufficient to demonstrate a causal relationship between tamoxifen and deep vein thrombosis, clinicians should be alert to the possibility of thromboembolic complications caused by tamoxifen when treating male infertility, even in the short term. Especially for patients at high risk of thromboembolism, physicians may need to consider other treatments.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81874472 and 81774315).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-630). The authors report grants from National Natural Science Foundation of China, during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- AinMelk Y, Belisle S, Carmel M, et al. Tamoxifen citrate therapy in male infertility. Fertil Steril 1987;48:113-7. [Crossref] [PubMed]
- Debbie Jiang MD, Alfred Ian Lee MD. Thrombotic Risk from Chemotherapy and Other Cancer Therapies. Cancer Treat Res 2019;179:87-101. [Crossref] [PubMed]
- Cosman F, Baz-Hecht M, Cushman M, et al. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res 2005;116:1-13. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics (Sao Paulo) 2013;68 Suppl 1:27-34. [Crossref] [PubMed]
- Patel DP, Chandrapal JC, Hotaling JM. Hormone-Based Treatments in Subfertile Males. Curr Urol Rep 2016;17:56. [Crossref] [PubMed]
- Chua ME, Escusa KG, Luna S, et al. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013;1:749-57. [Crossref] [PubMed]
- Moein MR, Tabibnejad N, Ghasemzadeh J. Beneficial effect of tamoxifen on sperm recovery in infertile men with nonobstructive azoospermia. Andrologia 2012;44 Suppl 1:194-8. [Crossref] [PubMed]
- Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. Journal of general internal medicine 2003;18:937-47. [Crossref] [PubMed]
- Lin HF, Liao KF, Chang CM, et al. Correlation of the tamoxifen use with the increased risk of deep vein thrombosis and pulmonary embolism in elderly women with breast cancer: A case-control study. Medicine 2018;97:e12842. [Crossref] [PubMed]
- Xu X, Chlebowski RT, Shi J, et al. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res Treat 2019;174:785-94. [Crossref] [PubMed]
- Allasia S, Motta G, Mirabelli M, et al. A case of deep vein thrombosis in a young male treated with tamoxifen for idiopathic infertility. Asian journal of andrology 2017;19:615-6. [Crossref] [PubMed]
- Cushman M, Costantino JP, Bovill EG, et al. Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial. British Journal of Haematology 2003;120:109-16. [Crossref] [PubMed]
- Erman M, Abali H, Oran B, et al. Tamoxifen induced tissue factor pathway inhibitor reduction: a clue for an acquired thrombophilic state? Ann Oncol 2004;15:1622-6. [Crossref] [PubMed]
- Rühl H, Schröder L, Müller J, et al. Tamoxifen induces resistance to activated protein C. Thromb Res 2014;133:886-91. [Crossref] [PubMed]
- Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e351S-418S.
- Liu D, Peterson E, Dooner J, et al. Diagnosis and management of iliofemoral deep vein thrombosis: clinical practice guideline. CMAJ 2015;187:1288-96. [Crossref] [PubMed]
- Hilleman DE, Razavi MK. Clinical and Economic Evaluation of the Trellis-8 Infusion Catheter for Deep Vein Thrombosis. J Vasc Interv Radiol 2008;19:377-83. [Crossref] [PubMed]