
Experience of diagnosis and management of metanephric adenoma: retrospectively analysis of 10 cases and a literature review
Introduction
Metanephric adenoma (MA), which derives from the embryonic tissue of the nephritic medulla, is a very rare benign tumor accounting for about 0.2–0.7% of adult renal epithelial neoplasms (1,2). To date, just a few hundred MA cases were reported in the literature and showed obvious female predominance (female: male =2:1). Clinical symptoms and signs of MA include abdominal mass, pain, hematuria, polycythemia and so on. However, most MA patients are asymptomatic and the lesions are always accidentally discovered in their health checkup by an ultrasound examination (3). Further abdominal computerized tomography (CT) or Magnetic Resonance Imaging (MRI) examination are essential for the diagnosis. Whereas, due to no specific imaging features or clinical manifestation compared with other renal malignancies, like Wilms tumor (WT) or papillary renal cell carcinoma (PRCC), the diagnosis of MA is always confirmed according to postoperative pathological results (4,5). What’s more, it is difficult to distinguish MA from some nephritic malignancy by intraoperative pathological diagnosis, while further postoperative pathology or immunohistochemistry can confirm the disease (6). Therefore, it brings challenges to urologists to make a definite diagnosis of MA and choose better surgical procedures for MA patients.
Due to the lack of sufficient awareness and experience of MA, it may lead to potential misdiagnosis and inadequate treatment. More experience is in demand to share with peers. Herein, we retrospectively analyzed the clinical characteristics, image features, therapeutic procedures, histological diagnosis and outcomes of ten MA cases treated in our hospital from 2010 to 2019. Moreover, we performed a brief literature review to the diagnosis and management of MA to provide a comprehensive understanding of this uncommon tumor.
We present the following article in accordance with the STROBE reporting checklist (7) (available at http://dx.doi.org/10.21037/tau-19-912).
Methods
Patients
A total of ten MA patients who were definitely diagnosed by postoperative pathology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to January 2019 were included in this study. We retrospectively summarized the experience of diagnosis and management of them. We summarized and compared the year and the gender, clinical symptoms, tumor sizes, results of imaging examination, results of pathological examination, surgical methods and outcomes of these patients.
All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and this study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2019-SR-312) and written informed consent for publication of the patients’ information and images was entirely obtained.
Imaging and pathological examination
All the ten cases received ultrasonography, as well as plain and enhanced CT scan. Five cases of them were further assessed by computed tomography angiography (CTA) examination, and two cases accepted MRI imaging. Intraoperative rapid pathology, postoperative routine histopathology and immunohistochemistry were used among all cases. Renal needle biopsy was adopted in two cases preoperatively.
Surgical treatment and follow-up
Laparoscopic partial nephrectomy (LPN) was performed in seven cases while laparoscopic radical nephrectomy (LRN) was selected for the other three cases. All patients acquired complete postoperative follow-up with regular evaluation of Ultrasonography and CT.
Statistical analysis
All the data were analyzed by Microsoft Excel, with a mean value and standard deviation (SD).
Results
Characteristics of subjects
Table 1 displayed the general characteristics of the ten cases. Nine females and one male were diagnosed with MA and treated in our hospital from January 2010 to January 2019. The average age of patients was 36.8 years (ranging from 11 to 67 years). Only in one case, the tumor was found in left kidney, while the other nine cases of tumors located in the right side. Additionally, almost all patients had no obvious clinical symptoms and admitted to hospital due to ultrasonography findings, except for one patient complained with acute flank pain and was found a 3 cm renal mass on a kidney with severe hydronephrosis, caused by a 2.5 cm renal stone by ultrasonography. What’s more, no polycythemia was found in any case. All patients have no specific family history.
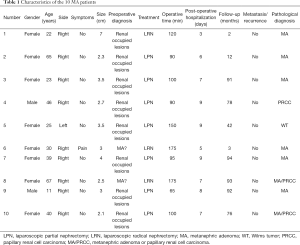
Full table
Imaging examination
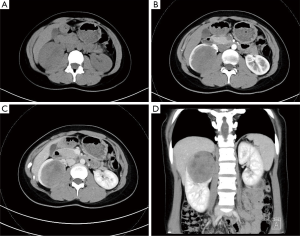
Plain and enhanced CT scan was the primary imaging examination of the ten MA patients. Eight of the tumors appeared as isodense (equal to the normal renal parenchyma) while two as hypodense (lower than the normal renal parenchyma) in CT plain. Dynamic contrast-enhanced CT revealed that enhanced degree of the tumors were all lower than that of the normal renal parenchyma. The average size of the tumors was 33.6±13.4 mm (range from 25 to 70 mm). Figure 1 exhibited the CT feature of the tumor from a 65-year-old woman. Plain CT showed a round homogeneous isopycnic mass while the enhanced CT showed a progressive enhanced low-density mass with a diameter of 2.3 cm. What’s more, Figure 2 displayed the CT finding of a 22-year-old girl with a tumor about 7 cm in diameter. The enhanced CT revealed a well-defined inhomogeneous mass with internal cystic degeneration and progressive enhancement both in cortex phase and parenchymal phase.

Surgical treatment
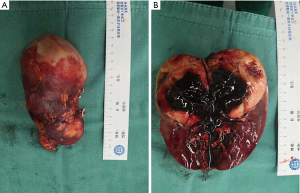
Minimally invasive laparoscopic operation was applied in all cases. LPN was performed in 7 cases and the other 3 cases underwent LRN. The average surgical time was 116±36 min (range from 65 to 175 mm). The average hospitalized days after surgery were 7±1.8 days (range from 3 to 9 days). No severe complications happened to patients. Figure 3 displayed the tumor in kidney excised by LRN of a 22-year-old girl, in the center of which revealed liquefactive necrosis.
Pathological characteristics
Postoperative routine pathology showed that all the tumors had entire capsules, and 7 cases were diagnosis as MA. While 2 cases were misdiagnosed as PRCC, and one case was misdiagnosed as WT. Microscopically, the morphology of tumor cells was uniform, with little eosinophilic cytoplasm and non-prominent nucleoli. Moreover, tumor cells showed tubular and acinar architecture, leading to the formation of glomerular-like or bud-like structures. Representative histopathology findings of the resect specimen of MA were shown in Figure 4.
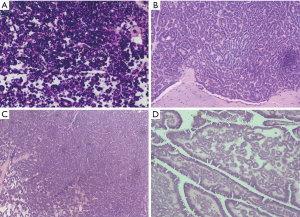
Further immunohistochemistry confirmed all cases. Most tumor cells were positive for WT-1(WT1 transcription factor), CD57 (beta-1,3-glucuronyltransferase 1), Vimentin, Ckpan, Pax-8 (paired box 8) and E-Cadherin while negative for NSE (neuron-specific enolase), CK7 (keratin 7), CD10 (membrane metalloendopeptidase), EMA (endosomal maturation defective) and AMACR (alpha-methylacyl-CoA racemase). Positive WT-1 and CD57, as well as negative CK7 and AMACR are characterized marks of MA. However, two cases exhibited a discordant immunophenotyped: one was negative for WT-1, another was positive for CK7.
Follow-up and prognosis
During a mean follow up of 58.3 month (3–94 months), all ten patients were alive with no local recurrences nor metastases confirmed by CT.
Discussion
The first case of MA was described as a bilateral and diffuse tumor by Bove et al. in 1979, who identified MA as a variant of nephroblastoma (8). In 1992, Brisigotti et al. officially named this unique type of renal tumor as MA, which was characterized by an unusual degree of differentiation and cell maturity and somewhat different from nephroblastoma via comparing one hundred childhood nephritic tumors (9). What’s more, Davis et al. summarized the clinicopathological characteristics of MA for the first time by retrospectively analyzing 50 cases (3). He found MA mainly occurred in females by well over 2:1 to males and were always unilateral, varying in size, well defined, noninvasive and might be associated with hemorrhage, cystic degeneration, and necrosis (3). Afterwards, accumulating cases were constantly reported from worldwide, providing us with profounder insights of this rare benign tumor (10-12). Nevertheless, the pathogenesis of MA remains unknown. Brown et al. found frequent chromosome 7 and 17 gain and sex chromosome loss by fluorescence in situ hybridization (FISH) analysis of MA, which might lead to genetic susceptibility (13). In addition, Choueiri et al. identified that BRAF V600E mutations are present in approximately 90% of all MA cases, which might serve as a promising diagnostic tool in the differential diagnosis of renal masses undergoing a percutaneous biopsy (14).
Despite that most MA cases were asymptomatic and accidentally found during physical examination, other potential symptoms of MA primarily included pain, hematuria, palpable mass (15). Besides, a higher incidence of polycythemia was reported in MA than in other renal tumor (16). All patients in our study were admitted to hospital by incidentally inspection other than one case who was accompanied with urologic calculi and hydronephrosis in the ipsilateral renal showed acute pain.
Ultrasound as the preferred check in urologic disease is essential for early detection of various renal tumor, although it can only roughly define the size, and whether the mass is solid or cystic (17). Further CT scan is the main imaging method for the diagnosis of MA. Plain CT usually shows an identical density of MA to the normal renal parenchyma. While in dynamic contrast-enhanced CT, the tumors are found to be slightly enhanced in the renal cortex, renal parenchymal, and pelvic phases and the enhanced degree is significantly lower than that of the normal renal parenchyma. Due to lack of blood supply, MA can be efficiently discriminate from the most common renal clear cell carcinoma which has an abundant blood supply and the enhancement pattern of rapid rise-rapid fall (4). However, it cannot be efficiently differentiated between MA and PRCC or WT by CT imaging. The typical MRI finding of MA is hypointense on T1WI and T2WI, which is not superior to enhanced CT and as a result, it is not imperative to perform a MRI examination (18). Only two cases in our study conducted MRI examination, but the images were unavailable. Overall in imaging manifestations, it is difficult to differentiate MA from some malignant renal tumor like WT or PRCC, which lead to limitation of preoperative diagnosis.
In this study, seven cases underwent LPN and showed no significant complication after surgery. A 22-year-old female and a 30-year-old female underwent LRN, respectively due to the huge size of tumor (6 cm × 7 cm × 6.1 cm) and a diseased kidney with severe hydronephrosis due to renal calculi. Another patient underwent LRN because the CT scan showed unclear boundaries of the tumor versus normal tissue, which suggested the malignancy potential of the tumor, as well as markedly atrophic kidney. No severe complications were observed in all patients. Besides, we carried out closely follow-up for these MA patients, and they all showed good prognoses with no recurrence or metastasis. Therefore, LPN is the pre-dominant surgical procedure to resect this benign tumor.
Postoperative histopathology and immunohistochemistry are most reliable methods for definite diagnosis of MA. In histopathology, these tumors are composed of small epithelial cells that form very small acini in an acellular stroma and also may form tubular, glomeruloid, or polypoid and papillary formations in a few cases (6,19). There is a certain rate of misdiagnosis of MA, because it is phenotypically similar to nephroblastoma and PRCC. In our study, postoperative routine pathology confirmed seven cases were MA, while two cases were misdiagnosed with PRCC and another was misdiagnosed with WT. More reliable diagnosis is depending on the further immunohistochemistry staining. In our cases, immunohistochemistry staining showed that neoplastic cells were always positive for WT-1, Vimentin, Ckpan, Pax-8 and E-Cadherin while negative for NSE, CK7, CD10, EMA and AMACR. The results revealed some differences from previously reported results, which reported that EMA was positive in MA (20). Positive WT-1 and CD57, as well as negative CK7 and AMACR are characterized marks of MA (21). However, two cases exhibited a discordant immunophenotyped: one was negative for WT-1, another was positive for CK7. Therefore, it is necessary to combine the histological structure and the results of immunohistochemistry for the diagnosis of MA. Recently, some scholars identified new potential biomarkers of MA such as BRAF V600E mutation (22). Ding et al. found that BRAF mutation was most frequent in thirty-six MA cases by next-generation DNA sequencing, and the BRAF V600E mutation also appeared in the MA cases, which was rare in other common renal tumors (23). As a result, emerging technologic would bring about more efficient diagnostic approach for MA.
It remains controversial whether performing a renal needle biopsy is necessary before operation. Blanco et al. suggested that preoperative recognition of MA by renal needle biopsy allowing for more conservative management, including partial nephrectomies or radiofrequency ablation (24). However, Guo et al. disapproved of preoperative biopsy because they thought it could lead to additional risk of tumor spread and bleeding, and could not change the surgical approach or planning whether MA or other malignancy (25). In our study, renal needle biopsy was applied in two cases and both suggested MA, whereas it could not entirely exclude the possibility of WT. The first patient who had biopsy is that 30-year-old women who suffered from acute flank pain and severe hydronephrosis caused by renal stone. Considering the patient was so young, we were going to gain more evidence about the tumorous type through preoperative biopsy to decide whether to perform LPN or not. But the patient herself finally gave up the choice of LPN. The other patient was the 67-year-old women whose CT scan showed unclear boundaries of the tumor versus normal tissue, which suggested the malignancy potential of the tumor, as well as markedly atrophic kidney. We did the biopsy for her to get more pathological evidence to choose LPN or not. But eventually, according to comprehensive judgement and to respect the patients’ wishes, both the two patients accepted LRP. While through preoperative biopsy, we can sometimes acquire basis for conservative treatment in selected patients.
Overall, MA is an extremely rare benign tumor which is worthy to report. However, surgery is inevitable due to the undefined preoperative diagnosis, or huge volume which might lead to compression of the renal parenchyma and the potential risk of internal necrosis. Fortunately, the prognosis of MA is optimistic. Nevertheless, more accurate diagnosis is helpful for the nephron sparing to a great extent. To a certain degree, several limitations of this paper should be considered. Firstly, the number of cases included in this study was limited. Meanwhile, the patients were regionally concentrated in the Jiangsu province of China. Therefore, more experience from peers from different areas and Medical Institutions will be helpful to the diagnosis and management of MA and to distinguish it from malignancies of the kidney.
Acknowledgments
Funding: This work was supported by the grant from National Natural Science Foundation of China (81600514), Six Talent Peak Project of High-level Talents in Jiangsu Province (WSW-017), 333 High-level Talents Training Project in Jiangsu Province, Qing Lan Project of Jiangsu University (JX2161015100), The Fifth Batch of Outstanding Young and Middle-aged Teachers’ Support Program of Nanjing Medical University, Professional from Six-Pronged Top-Talent Program (LGY2018053), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231802).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-19-912
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-19-912
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-912). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and this study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2019-SR-312) and written informed consent for publication of the patients’ information and images was entirely obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 2002;26:281-91. [Crossref] [PubMed]
- Kuroda N, Tol M, Hiroi M, et al. Review of metanephric adenoma of the kidney with focus on clinical and pathobiological aspects. Histol Histopathol 2003;18:253-7. [PubMed]
- Davis CJ Jr, Barton JH, Sesterhenn IA, et al. Metanephric adenoma. Clinicopathological study of fifty patients. Am J Surg Pathol 1995;19:1101-14. [Crossref] [PubMed]
- Yan J, Cheng JL, Li CF, et al. The findings of CT and MRI in patients with metanephric adenoma. Diagn Pathol 2016;11:104. [Crossref] [PubMed]
- Lerut E, Roskams T, Joniau S, et al. Metanephric adenoma during pregnancy: clinical presentation, histology, and cytogenetics. Hum Pathol 2006;37:1227-32. [Crossref] [PubMed]
- Mantoan Padilha M, Billis A, Allende D, et al. Metanephric adenoma and solid variant of papillary renal cell carcinoma: common and distinctive features. Histopathology 2013;62:941-53. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Bove KE, Bhathena D, Wyatt RJ, et al. Diffuse metanephric adenoma after in utero aspirin intoxication. A unique case of progressive renal failure. Arch Pathol Lab Med 1979;103:187-90. [PubMed]
- Brisigotti M, Cozzutto C, Fabbretti G, et al. Metanephric adenoma. Histol Histopathol 1992;7:689-92. [PubMed]
- Li G, Tang Y, Zhang R, et al. Adult metanephric adenoma presumed to be all benign? A clinical perspective. BMC Cancer 2015;15:310. [Crossref] [PubMed]
- Hes O, Curik R, Malatkova V, et al. Metanephric adenoma and papillary carcinoma with sarcomatoid dedifferentiation of kidney. A case report. Pathol Res Pract 2003;199:629-32. [Crossref] [PubMed]
- McNeil JC, Corbett ST, Kuruvilla S, et al. Metanephric adenoma in a five-year-old boy presenting with chyluria: case report and review of literature. Urology 2008;72:545-7. [Crossref] [PubMed]
- Brown JA, Anderl KL, Borell TJ, et al. Simultaneous chromosome 7 and 17 gain and sex chromosome loss provide evidence that renal metanephric adenoma is related to papillary renal cell carcinoma. J Urol 1997;158:370-4. [Crossref] [PubMed]
- Choueiri TK, Cheville J, Palescandolo E, et al. BRAF mutations in metanephric adenoma of the kidney. Eur Urol 2012;62:917-22. [Crossref] [PubMed]
- Hartman DJ, Maclennan GT. Renal metanephric adenoma. J Urol 2007;178:1058. [Crossref] [PubMed]
- Wang P, Tian Y, Xiao Y, et al. A metanephric adenoma of the kidney associated with polycythemia: A case report. Oncol Lett 2016;11:352-4. [Crossref] [PubMed]
- Hu YC, Wu L, Yan LF, et al. The imaging features of metanephric adenoma: a case report and review of literature. Onco Targets Ther 2015;8:445-9. [PubMed]
- Bastide C, Rambeaud JJ, Bach AM, et al. Metanephric adenoma of the kidney: clinical and radiological study of nine cases. BJU Int 2009;103:1544-8. [Crossref] [PubMed]
- Schmelz HU, Stoschek M, Schwerer M, et al. Metanephric adenoma of the kidney: case report and review of the literature. Int Urol Nephrol 2005;37:213-7. [Crossref] [PubMed]
- Muir TE, Cheville JC, Lager DJ. Metanephric adenoma, nephrogenic rests, and Wilms' tumor: a histologic and immunophenotypic comparison. Am J Surg Pathol 2001;25:1290-6. [Crossref] [PubMed]
- Kinney SN, Eble JN, Hes O, et al. Metanephric adenoma: the utility of immunohistochemical and cytogenetic analyses in differential diagnosis, including solid variant papillary renal cell carcinoma and epithelial-predominant nephroblastoma. Mod Pathol 2015;28:1236-48. [Crossref] [PubMed]
- Chan E, Stohr BA, Croom NA, et al. Molecular characterization of metanephric adenomas beyond braf: genetic evidence for potential malignant evolution. Histopathology 2020;76:1084-90. [Crossref] [PubMed]
- Ding Y, Wang C, Li X, et al. Novel clinicopathological and molecular characterization of metanephric adenoma: a study of 28 cases. Diagn Pathol 2018;13:54. [Crossref] [PubMed]
- Blanco LZ, Schein CO, Patel T, et al. Fine-needle aspiration of metanephric adenoma of the kidney with clinical, radiographic and histopathologic correlation: a review. Diagn Cytopathol 2013;41:742-51. [Crossref] [PubMed]
- Guo J, Zhou X, Fu B, et al. Retroperitoneal laparoscopic partial nephrectomy for treatment of metanephric adenoma (Report of 6 cases). Springerplus 2016;5:996. [Crossref] [PubMed]


