The vasal fluid proteomic profile and microscopic sperm presence at time of vasectomy reversal
Introduction
Every year in the United Sates it is estimated that over 500,000 men undergo vasectomy (1), and due to various reasons, up to 6% of these patients eventually elect to undergo vasectomy reversal (VR) (2). At the time of VR, microsurgeons evaluate both the gross and microscopic appearance of vasal fluid to determine the likelihood of secondary vasal obstruction, which influences the type of surgery to be performed and guides clinical decision making after surgery (3).
Although also common practice, consideration of the gross appearance of vasal fluid has not been significantly correlated with success of VR (4,5). However, if the fluid’s gross appearance is clear and copious, then vasovasostomy (VV) is routinely performed, even in the absence of sperm (6). When whole sperm are identified, the patency rate after VV approaches 99.5% (7). The Vasovasostomy Study Group first described microscopic fluid findings, obstructive interval, and presence of sperm granuloma were associated with pregnancy rates in 1991 (8) and more recent studies have contributed to this body of evidence (9,10); However, few other factors have been identified to guide practice since this time.
Over the past decade, the sperm and seminal fluid proteomes have been largely defined, including a number of proteins with altered expression in men with semen abnormalities or poorly functioning sperm (11). Several protein biomarkers have been studied as candidate biomarkers of obstructive azoospermia or to be used as a confirmatory test for post vasectomy clearance (12-14). However, the proteome of vasal fluid in post-vasectomy patients remains uncharacterized, and it is unclear if this fluid is primarily derived from the epididymis, vas deferens, or sperm.
Understanding the origins of this fluid may explain factors contributing to obstruction following VR, or the origins of post vasectomy pain syndrome. The ability to identify and correlate a sperm, testis or epididymis specific protein with patency and pregnancy rates would allow surgeons save time in the operative theater, make more informed management decisions and better counsel patients on expected outcomes. In addition, identifying a biomarker protein to delineate non-obstructive azoospermia (NOA) versus obstructive azoospermia (OA) will allow for optimal counseling and treatment of these patients compared to the current paradigm.
This study characterizes the proteomic profile of vasal fluid at time of VR and compared these findings to the gross and microscopic appearance of the fluid at this time. It also compares the proteomic profiles to the patients’ associated patency and fertility outcomes. It was hypothesized that testis, epididymal and sperm specific proteins would be relatively less abundant in samples with no sperm present on microscopy. The following article is presented in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-703).
Methods
A prospective cohort study at a single academic center was conducted after approval by from the Oregon Health and Science University Institutional Review Board (FWA00000161; IRB00000471) and all patients provided informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients who underwent VR for pain or fertility reasons were included, while patients who did not provide permission, who had suspected NOA or who’s bilateral samples could not be properly collected, stored or processed were excluded. In men who underwent unilateral VR for pain a unilateral sample was collected. Vasal fluid samples at time of VR were collected from consecutive patients post-vasectomy between the years 2015 to 2016. The patent’s age, obstructive interval, indication for procedure, and procedure performed [VV or Vasoepididymostomy (VE)] were recorded. The patient’s status of patency and pregnancy at time of follow up were also analyzed. Postoperative patency was defined as the presence of motile sperm on at least one semen analysis and clinical pregnancies were determined by either review of electronic medical records or phone survey response.
During the VR samples were expressed from the cut end of the vas and collected in sterile glass capillary tubes. In patients who underwent VE, additional fluid from the epididymis was not analyzed. The gross appearance of the sample was quantified as clear, opalescent or creamy/pasty. The microscopic appearance of the fluid was quantified as having motile sperm, non-motile whole sperm, sperm parts, or no sperm. For analysis, samples were grouped into one of two groups: microscopic presence of whole sperm (either motile or non-motile) and/or sperm parts versus no sperm on microscopy at time of VR. We determined these groupings would be most likely to detect a difference in sperm, testis and epididymal derived proteins based on current understanding of the post vasectomy obstructive process. Vasal samples were also taken from patients at time of vasectomy for controls in a similar fashion and run in a discovery analysis to optimize sample preparation. These samples were not included in the final analysis.
Statistical analysis
Researchers completing the proteomic and statistical analysis of the fluids were blinded to the gross and microscopic characteristics of the samples to prevent bias. For proteomic and statistical analysis, all samples were extruded from sterile collection tubes, dispersed in buffer, a protein assay performed, samples reduced/alkylated, and proteins digested by overnight incubation with trypsin. Proteomic profiles were generated using liquid chromatography/ tandem mass spectrometry as previously described (15), except using a single dimensional chromatographic separation with 90 min of data collection and 10 µg of peptide injected per sample. Proteins were identified using a human swissprot database (UniProt Consortium) and Comet search engine (16), while controlling protein false discovery rate (FDR) at or below 1.2% as previously described (17). Identified proteins were then categorized by Gene Ontology (GO) terms and numbers of assigned MS/MS spectra (spectral counts) to each protein used to estimate differences in relative protein abundance (18). Differential Expression was assessed using Bioconductor edgeR package (Bioconductor Open Source Software for Bioinformatics) with a multiple-testing correction included to control the FDR for test of differential protein abundance as previously described (19). Recruitment, collection and analysis was stopped after interim analysis demonstrated our current results supporting the null hypothesis.
Results
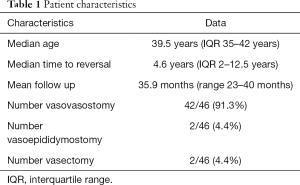
A total of 46 vasal fluid samples from 25 patients were collected. Of patients enrolled, 56% (14/25) underwent VR for fertility reasons, while 36% (9/25) underwent VR for pain and 8% (2/25) were vasectomy controls. Patient characteristics can be seen in Table 1. Median patient age was 39.5 years old (IQR 35–42 years old). Median time to VR, or the mean obstructive interval, was 4.6 years (IQR 2–12.5 years). Patients were followed in our andrology clinic with a mean follow up time of 35.9 months (range, 23–40 months). Regarding the gross appearance of vasal fluid at time of VR, 29.5% (13/44) of samples had clear fluid, 47.7% (21/44) had opalescent fluid and 22.7% (10/44) had creamy/pasty fluid. With respect to microscopic appearance of samples, motile whole sperm were present in 36% of samples, non-motile whole sperm were present in 41% of samples and sperm parts were present in 7%. Thus, 84% of samples contained sperm or sperm parts. Sperm and/or sperm parts were absent in only 16% of samples. Based on the surgeon’s clinical decision making paradigm 91.3% (42/46) of testicular units underwent VV and 4.4% (2/46) of testicular units underwent VE. The remaining 4.4% (2/46) of testicular units were vasectomy controls.
Full table
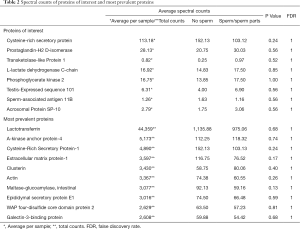
On proteomic analysis, a total of 1,692 proteins were identified by at least two unique peptides and at a FDR of 1.2% (please see supplementary files for complete list of proteins detected, GO terms, pathway reports, and edgeR analysis Available at: https://cdn.amegroups.cn/static/application/9031b1c97015ed6de67ccf8cbfc0b5fe/tau-20-703-01.xlsx; https://cdn.amegroups.cn/static/application/deec701c85c116f07d7ab63fc4a5181b/tau-20-703-02.xlsx). Excluding major serum or erythrocyte proteins introduced by blood contamination during surgery, the top 10 vasal proteins by abundance were: Lactotransferrin, A-kinase anchor protein 4, Cysteine-rich secretory protein 1, Extracellular matrix protein 1, Clusterin, Actin, Maltase-glucoamylase, Epididymal secretory protein E1, WAP four-disulfide core domain protein 2, and Galectin-3-binding protein (Table 2). Dynein proteins were relatively absent in all samples, while maltase-glucoamylase, a transmembrane intestinal protein was relatively abundant.
Full table
Numerous human secretory and inflammatory proteins were detected in all samples, with no significant difference based on the gross appearance of the sample. Significant differences were observed in sperm derived proteins between clear versus creamy samples, with epididymal sperm binding protein 1, clathrin heavy chain 1, and fatty acid synthase increased in creamy samples (FDR <0.047). No significant differences in proteomic profile were observed between clear versus opalescent samples.
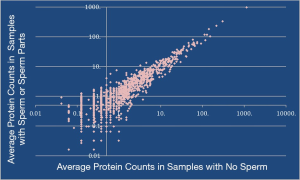
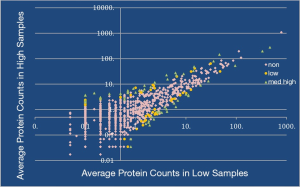
When samples were grouped based on microscopic presence of sperm or sperm parts compared to no sperm there was no significant difference between relative spectral counts (Figure 1). The reports of several proteins of interest can be seen in Table 2. Samples were then collated with regards to abundance of 5 previously published obstruction specific proteins and this demonstrated 272 significantly different proteins between the two groups (Figure 2).
With regards to clinical outcomes, 15 of 15 patients (100%) who had a postoperative semen analysis demonstrated patency, as defined by millions of intact motile sperm present. Of 10 patients who reported to have attempted to achieve natural pregnancy 4 (40%) were successful. Combining these patency and pregnancy rates allowed us to account to 17 of 23 patients (74%) to be confirmed surgically successful. There was no correlation between patency rate or obstructive interval and proteomic profile.
Discussions
The proteomic profile of vasal fluid at time of VR has not been reported previously. The majority of vasal proteins detected are known proteins found in sperm (20,21) or secreted from the epididymis (22). The vasal fluid protein composition varied significantly despite vasectomy and obstruction. This study found that sperm, testis and epididymis derived proteins were present in samples even despite the microscopic absence of sperm or sperm parts. The relative absence of dynein proteins was unexpected and may indicate the rapid degradation of this flagellar protein following vasectomy.
One of the more abundant proteins found, a transmembrane intestinal protein, Maltase-glucoamylase, suggests degradation of the vas deferens membrane components following vasectomy. Findings of elevated levels of this protein may be associated with the gross appearance of vasal fluid secondary to obstruction, although due to our small sample size we were not able to identify a correlation. Reports have found that vasal fluid produced between two obstructed vas segments in patients with segmental dysplasia of the vas deferens to be thick, white, toothpaste-like material similar to that in men with secondary epididymal obstruction. Thus, it has been concluded that this vasal “toothpaste” must be derived from vasal epithelium, not sperm (23,24).
This study found that gross fluid appearance was associated with increased levels of several known sperm proteins in creamy samples when compared to clear samples. The variation in protein abundance of opalescent fluid was too great to detect significant differences compared to clear fluid. This is consistent with knowledge that pasty fluid rarely has any sperm or sperm parts seen (25,26). As identification of whole spermatozoa or sperm parts in the vasal fluid at the time of VV has been positively associated with post-operative patency (5,27), identifying these proteins may help decipher the changes sperm undergo after vasectomy. Further understanding of these sperm proteins will lead to better understanding of the obstructive process on sperm degradation, or may also be correlated with patency and outcomes.
Although the current clinical application of sperm and seminal fluid proteomics is limited, several studies have been conducted comparing the seminal proteomes of men with OA to those with NOA in attempts to identify potential biomarkers for obstruction (12,13,28). Cysteine-rich secretory protein 1 (CRISP-1) has been proposed as a biomarker of obstructive infertility, as one study found all seminal plasma samples from normospermic and NOA donors were CRISP-1 positive, whereas CRISP-1 was absent or present at low levels in samples from patients with OA (29). Another protein of interest is lipocalin-type prostaglandin D synthase (L-PGDS), as when levels were compared in men with normal semen parameters, OA, NOA, and in vasectomized men, seminal L-PGDS levels were significantly lower in men with OA (11). This study detected high levels of CRISP-1 in our samples regardless of microscopic sperm presence and we suspect levels of this protein may be altered in the process of post-vasectomy obstruction and patency. This may be due to the low rate of more proximal obstruction confirmed by a high number of patients in this study that underwent successful VR. Evaluating a higher number of obstructed patients may potentially detect a difference in biomarker proteins.
Numerous human secretory and inflammatory proteins were also detected in all samples. Although little is known about the disease process of post vasectomy pain syndrome (PVPS), it has been demonstrated that VR, either by VV or VE, can provide long-term relief from PVPS (30). Future studies are required to determine if elevated levels of these proteins are associated with PVPS and could lead to better understanding of management of this disease.
Limitations of this study include a small sample size and a limited heterogeneity of samples. This study may be improved upon by vastly increasing sample size, as there was large variability between the relative abundance of proteins in each sample. An improved heterogeneity of patients may also help detect potential biomarker proteins, as proximal obstruction was low in this patient population with only 4.4% undergoing VE based on current clinical decision making paradigms and 74% of patients demonstrating patency during the follow up period. The proteomic analysis conducted was a discovery analysis and could be more accurately analyzed with respect to specific candidate proteins in the future. Continued study of the vasal fluid proteome will contribute to a better understanding of outcomes following VR and will improve the management of OA.
Conclusions
This study characterized the proteomic profile and most abundant proteins present in vasal fluid at time of VR. The vasal fluid protein composition varies significantly despite sperm presence on light microscopy. Further evaluation is needed to determine if a potential protein biomarker is best related to sperm presence or could be used to better predict VR fertility outcomes.
Acknowledgments
We would like to acknowledge Jennifer Cunliffe, PhD, a research associate with The Proteomics Shared Resource at Oregon Health and Science University for her time and efforts with processing samples and data collection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-703
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-703
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-703). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by from the Oregon Health and Science University Institutional Review Board (FWA00000161; IRB00000471) and all patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ostrowski KA, Holt SK, Haynes B, et al. Evaluation of Vasectomy Trends in the United States. Urology 2018;118:76-9. [Crossref] [PubMed]
- Sandlow JI, Nagler HM. Vasectomy and vasectomy reversal: important issues. Preface. Urol Clin North Am 2009;36:xiii-xxiv. [Crossref] [PubMed]
- Hayden RP, Li PS, Goldstein M. Microsurgical vasectomy reversal: contemporary techniques, intraoperative decision making, and surgical training for the next generation. Fertil Steril 2019;111:444-53. [Crossref] [PubMed]
- Ostrowski KA, Polackwich AS, Conlin MJ, et al. Impact on Pregnancy of Gross and Microscopic Vasal Fluid during Vasectomy Reversal. J Urol 2015;194:156-9. [Crossref] [PubMed]
- Ramasamy R, Mata DA, Jain L, et al. Microscopic visualization of intravasal spermatozoa is positively associated with patency after bilateral microsurgical vasovasostomy. Andrology 2015;3:532-5. [Crossref] [PubMed]
- Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am 2009;36:375-82. [Crossref] [PubMed]
- Goldstein M, Li PS, Matthews GJ. Microsurgical vasovasostomy: the microdot technique of precision suture placement. J Urol 1998;159:188-90. [Crossref] [PubMed]
- Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 1991;145:505-11. [Crossref] [PubMed]
- Kolettis PN, Burns JR, Nangia AK, et al. Outcomes for vasovasostomy performed when only sperm parts are present in the vasal fluid. J Androl 2006;27:565-7. [Crossref] [PubMed]
- Smith RP, Khanna A, Kovac JR, et al. The significance of sperm heads and tails within the vasal fluid during vasectomy reversal. Indian J Urol 2014;30:164-8. [Crossref] [PubMed]
- Barazani Y, Agarwal A, Sabanegh ES Jr. Functional Sperm Testing and the Role of Proteomics in the Evaluation of Male Infertility. Urology 2014;84:255-61. [Crossref] [PubMed]
- Heshmat SM, Mullen JB, Jarvi KA, et al. Seminal plasma lipocalin-type prostaglandin D synthase: a potential new marker for the diagnosis of obstructive azoospermia. J Urol 2008;179:1077-80. [Crossref] [PubMed]
- Batruch I, Smith CR, Mullen BJ, et al. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J Proteome Res 2012;11:1503-11. [Crossref] [PubMed]
- Korbakis D, Schiza C, Brinc D, et al. Preclinical evaluation of a TEX101 protein ELISA test for the differential diagnosis of male infertility. BMC Med 2017;15:60. [Crossref] [PubMed]
- Shang F, Wilmarth PA, Chang ML, et al. Newborn mouse lens proteome and its alteration by lysine 6 mutant ubiquitin. J Proteome Res 2014;13:1177-89. [Crossref] [PubMed]
- Eng JK, Jahan TA, Hoopmann MR. Comet: an open-source MS/MS sequence database search tool. Proteomics 2013;13:22-4. [Crossref] [PubMed]
- Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Infor 2009;2:223-34. [Crossref] [PubMed]
- Krey JF, Wilmarth PA, Shin JB, et al. Accurate label-free protein quantitation with high- and low-resolution mass spectrometers. J Proteome Res 2014;13:1034-44. [Crossref] [PubMed]
- Smith JR, David LL, Appukuttan B, et al. Angiogenic and Immunologic Proteins Identified by Deep Proteomic Profiling of Human Retinal and Choroidal Vascular Endothelial Cells: Potential Targets for New Biologic Drugs. Am J Ophthalmol 2018;193:197-229. [Crossref] [PubMed]
- Bayram HL, Claydon AJ, Brownridge PJ, et al. Cross-species proteomics in analysis of mammalian sperm proteins. J Proteomics 2016;135:38-50. [Crossref] [PubMed]
- Skerget S, Rosenow MA, Petritis K, et al. Sperm Proteome Maturation in the Mouse Epididymis. PLoS One 2015;10:e0140650. [Crossref] [PubMed]
- Turner TT, Riley TA, Mruk DD, et al. Obstruction of the Vas Deferens Alters Protein Secretion by the Rat Caput Epididymidal Epithelium In Vivo. J Androl 1999;20:289-97. [PubMed]
- Anger JT, Goldstein M. Intravasal "toothpaste" in men with obstructive azoospermia is derived from vasal epithelium, not sperm. J urol 2004;172:634-6. [Crossref] [PubMed]
- Saitz TR, Thomas AA. Unilateral segmental dysplasia of the vas deferens. Can J Urol 2018;25:9620-2. [PubMed]
- Belker AM, Konnak JW, Sharlip ID, et al. Intraoperative observations during vasovasostomy in 334 patients. J Urol 1983;129:524-7. [Crossref] [PubMed]
- Sharlip ID, Belker AM, Konnak JW, et al. Relationship of gross appearance of vas fluid during vasovasostomy to sperm quality, obstructive interval and sperm granuloma. J Urol 1984;131:681-3. [Crossref] [PubMed]
- Scovell JM, Mata DA, Ramasamy R, et al. Association Between the Presence of Sperm in the Vasal Fluid During Vasectomy Reversal and Postoperative Patency: A Systematic Review and Meta-analysis. Urology 2015;85:809-13. [Crossref] [PubMed]
- Yamakawa K, Yoshida K, Nishikawa H, et al. Comparative analysis of interindividual variations in the seminal plasma proteome of infertile men with identification of potential markers for azoospermia in infertile patients. J Androl 2007;28:858-65. [Crossref] [PubMed]
- Légaré C, Cloutier F, Makosso-Kallyth S, et al. Cysteine-rich secretory protein 1 in seminal plasma: potential biomarker for the distinction between obstructive and nonobstructive azoospermia. Fertil Steril 2013;100:1253-60. [Crossref] [PubMed]
- Polackwich AS, Tadros NN, Ostrowski KA, et al. Vasectomy Reversal for Postvasectomy Pain Syndrome: A Study and Literature Review. Urology 2015;86:269-72. [Crossref] [PubMed]