
LncRNA CARLo-7 facilitates proliferation, migration, invasion, and EMT of bladder cancer cells by regulating Wnt/β-catenin and JAK2/STAT3 signaling pathways
Introduction
Bladder cancer (BC) is one of the top ten most frequently occurred cancer in the world, with nearly 150,000 deaths and 400,000 new cases yearly (1,2). Etiology studies indicate that cigarette smoking and various occupational exposure are the most important risk factors for bladder cancer, and other non-environmental risk factors including age, sex, ethnicity, body weight, lifestyle and family history (3). Whole-genome sequencing studies of bladder cancer identifies recurrent mutations in TP53, RB1, TSC1, FGFR3 and PIK3CA genes, and amplification or deletion of PPARG, E2F3, EGFR, CCND1, MDM2 and CDKN2A genes, revealing the molecular pathogenesis of bladder cancer (4). According to genetic alternations, cell origin and tumor invasion depth, bladder cancer can be classified into non-muscle invasive bladder cancer (NMIBC) or muscle-invasive bladder cancer (MIBC). Among these two subtypes, NMIBC occurs more often but with a low tendency to progress, while MIBC is more lethal with a high rate of metastasis. Transurethral resection and following chemotherapy or bacille Calmette-Guérin (BCG) adjuvant immunotherapy are current treatment options for NMIBC, while radical cystectomy and following adjuvant chemotherapy and radiation therapy are frequently used in MIBC. Recent progress in bladder cancer targeted therapy or immunotherapy such as using of Lapatinib or anti-PD1/PDL-1 mAb hold promising potential, however the effect is limited and there is no apparent change in the 5-year survival rate of MIBC patients due to the highly metastatic competency and heterogeneous lineages of cancer cells (5-7). Therefore, it is vital to understand the molecular mechanisms of BC development, find new therapeutic targets, and develop new regimens for BC treatment.
Long noncoding RNAs (lncRNAs) are non-protein-coding RNA transcripts longer than 200 nucleotides. They are suggested to play pivotal functions in multiple biological processes, including cell differentiation, proliferation, autophagy, and apoptosis, by regulating the expression of different genes. Indeed, lncRNAs are also proved to involve in the development and progressing of many human diseases, including bladder cancer (8). Cancer-associated region long noncoding RNAs (CARLos) are newly discovered lncRNAs in the 8q24.21 region of human genome, including CARLo-7. Recently study indicates CARLo-7 is the only bladder cancer specific lncRNA in the CARLos cluster, and high CARLo-7 expression is correlated with advanced tumor stage (9). Moreover, CARLo-7 (also known as CASC11, LINC00990 and MYMLR) has been demonstrated to play a role in the tumorigenesis of various cancers. In gastric cancer, CARLo-7 is highly expressed, while silencing CARLo-7 suppresses proliferation and induces apoptosis and cell cycle arrest (10). In ovarian cancer, CARLo-7 overexpression facilitates cell proliferation and inhibits apoptosis through regulating miR-182, and high CARLo-7 expression predicts poor prognosis of ovarian cancer patients (11). Up-regulated CARLo-7 is also found in lung cancer tissues and cell lines, and silencing CARLo-7 represses cell proliferation through miR-302/CDK1 axis (12). Furthermore, CARLo-7 is involved in the maintaining of oncogenic MYC transcriptional activity and cell cycle progression though in low expression level, suggesting an important role of CARLo-7 in MYC-related tumorigenesis (13).
Accumulated studies reveal the importance of Wnt/β-catenin and JAK2/STAT3 signaling in human cancers. For example, Wnt/β-catenin signaling plays a central role in the development of bladder cancer as well as the maintenance of adult urothelial tissue homeostasis (14). In addition, JAK2/STAT3 signaling is reported to involve in the regulating of migration, invasion and metastasis of bladder cancer cells (15,16). More importantly, CARLo-7 is reported to activate Wnt/β-catenin signaling and promote proliferation and metastasis of colon cancer cells (17). Thus, we focused on the activation of Wnt/β-catenin and JAK2/STAT3 signaling pathways in our study. Though recent reports suggest that CARLo-7 is bladder cancer specific lncRNA and holds prognostic value, and high CARLo-7 expression promotes proliferation of bladder cancer cells (9), the exact biological functions and underlying mechanisms of CARLo-7 in bladder cancer are still to be resolved. Thus, our study is aimed to explore the role of CARLo-7 in bladder cancer and the potential mechanisms involved. We found CARLo-7 overexpression facilitated the proliferation of bladder cancer cells while silencing CARLo-7 induced apoptosis and suppressed metastasis and EMT of bladder cancer cells. Furthermore, Wnt/β-catenin and JAK2/STAT3 signaling pathways were involved in the regulating of proliferation, migration, invasion and EMT of bladder cancer cells by CARLo-7. Therefore, CARLo-7 might be a potential therapeutic target for bladder cancer treatment. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1293).
Methods
Patients and tissue samples
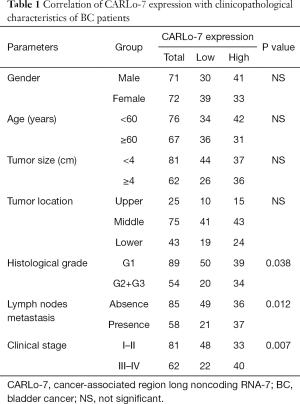
Written informed consent was obtained from all patients enrolled in this study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). A total of 143 BC samples and paired adjacent normal tissues were collected from Peking Union Medical College Hospital between March 2015 and April 2018. The inclusion criteria were defined as certain diagnosis of bladder cancer. Also, patients who had received any treatment such as chemotherapy, radiotherapy, and biological medication (monoclonal antibodies) before the sampling were excluded from the study. The collected samples were at once frozen in liquid nitrogen and stored at −80 °C for future use. The Ethics Committee approved this study of Qianfoshan Hospital Affiliated to Shandong University, SYXK (Jing) 2017-0015. The clinical features of the enrolled patients were shown in Table 1.
Full table
Cell culture
Human BC cell lines T24 and HT1197, and human epithelial SV40 immortalized uroepithelium cell line SV-HUC-1 were all obtained from the Stem Cell Bank, Chinese Academy of Sciences, Shanghai, China. Cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) and 1% PenStrep (100 U/mL Penicillium and 100 µg/mL Streptomycin) in a humid atmosphere containing 5% CO2 at 37 °C.
Quantitative real-time polymerase chain reaction (qRT-PCR)
TRIzol® reagent (Invitrogen, Thermo Fisher Scientific, USA) was used to extract total RNA from cultured cells, and tissue samples as protocol showed. PrimeScript RT Reagent kit synthesized complementary DNA (Takara Bio, Inc., Otsu, Japan). RT-PCR was performed using the SYBR Prime Script™ RT-qPCR kit (Takara Bio, Inc., Otsu, Japan) on an ABI 7500 system (Applied Biosystems, Thermo Fisher Scientific, USA). The 2−ΔΔCT method was used for calculation, and each sample was done in triplicate. The primer sequences were listed below: CARLo-7, forward: 5'-GCTGC AGAAG GTCCG AAGAA-3', reverse: 5'-TTCAC CACGT CCAGT TGCTT-3'. GAPDH, forward: 5'-GGAAA GCTGT GGCGT GAT-3', reverse: 5'-AAGGT GGAAG AATGG GAGTT-3'.
Plasmids and transfection
Short-hairpin RNAs targeting CARLo-7 (sh-CARLo-7) or a non-targeting sequence control (sh-NC) was cloned into the U6/GFP/Neo plasmid (GenePharma, Shanghai, China). Sh-RNA sequences against CARLo-7 are shown as follows: sh-CARLo-7: 5'-GCCCA CATCA AGCCT TCAT-3', sh-NC: 5'-ACGGA GGCTA AGCGT CGCAA-3'. CARLo-7 expression plasmid (pEX-CARLo-7) was constructed by cloning the full length of CARLo-7 into pEX-2 (GenePharma, Shanghai, China). The pEX-2 empty vector was used as control (pEX-NC). Primers used for cloning full length CARLo-7 were: forward, 5'-TCTTT CAGGT TGGCT GCAGA-3' reverse, 5'-ACCTT CTGCT AGGGT CTGAGA-3'. Cell transfection was conducted using lipofectamine 3000 reagents (Life Technologies Corporation, Carlsbad, CA, USA) as protocol showed. Cells were used for other experiments 48 hours post-transfection.
Cell viability assay
Cell viability was measured by the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega #G7572), as the protocol showed. Briefly, the cells and CellTiter-Glo reagents were balanced to room temperature for 30 minutes. Then remove the culture medium, add the CellTiter-Glo reagents (100 µL per well in 96-well plates) into the well, and mix thoroughly on an orbital shaker. Incubate the plate at dark for 10 minutes, then record the luminescence signal on a microplate reader.
BrdU assay
Cells were seeded in 6-well plates for 24 hours, then incubated with BrdU (10 µmol/L) for 4 hours at 37 °C. Then stained the cells with BrdU Mouse mAb (Cell signaling #5292, 1:1,000) at room temperature for 1.5 hours. Anti-mouse IgG (Alexa Fluor 488 Conjugate) (Cell signaling #4408, 1:500) was used as the secondary antibody. DAPI (Invitrogen, USA) was used to stain the nucleus. Cells were mounted LSM 5 Pa Laser Scanning Microscope (Zeiss Germany, Oberkochen, Germany).
Transwell cell migration and invasion
Seeded the cells (1×106/well) in the upper chamber (Costar Corp, USA) without serum, then filled the lower chamber with a medium holding 20% FBS. Then cells were cultured for 48 hours, and migration cells were fixed by 4% paraformaldehyde for 15 minutes and stained with crystal violet. Cell invasion was evaluated by pre-coating the filter with Matrigel (BD Biosciences, USA), then allowed the cells to invasion through the filter for 48 hours and stained with Giemsa dye. All experiments were conducted in triplicates.
Flow cytometry
Cells were digested with 0.05% trypsin, then washed three times with phosphate-buffered saline (PBS). Then resuspended 1×106 cells with binding buffer and incubated with 5 µL Annexin V-FITC and 10 µL propidium iodide (PI) (Sigma-Aldrich, USA) for 15 minutes at room temperature, avoiding night. Flow cytometry recorded the fluorescence signal.
Western blotting
Cell lysates were collected by digesting with RIPA buffer (Beyotime, Nanjing, China) and protease inhibitors (Sigma-Aldrich, USA). BCA kit determined protein concentration (Thermo Fisher, USA). Thirty µg protein were loaded on 10% or 15% SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes were incubated with specific first antibodies and corresponding second antibody. Primary antibodies purchased from Abcam (Cambridge, UK) included anti-E-cadherin (ab182733, 1:1,000), anti-N-cadeherin (ab13847, 1:500), anti-Vimentin (ab44976, 1:2,000), anti-JAK2 (ab108596, 1:5,000), anti-p-JAK2 (ab32101, 1:5,000), anti-STAT3 (ab31369, 1:1,000), anti-p-STAT3 (ab30645, 1:1,000), anti-β-catenin (ab6302, 1:4,000) and anti-β-actin (ab8245, 1:1,000). Secondary antibodies included goat anti-mouse IgG (ab6789, 1:5,000; Abcam) and goat anti-rabbit IgG (ab6721, 1:5,000; Abcam).
Statistical analysis
Data were analyzed by GraphPad Prism 8.0 software (GraphPad Inc., USA). The difference between the two groups was analyzed by paired sample t-test. The difference between multiple groups was analyzed by one-way analysis. All data were shown as mean ± standard deviation (
Results
LncRNA CARLo-7 was upregulated in BC tissues and correlated with poor prognosis
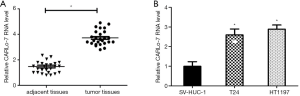
To explore the potential role of CARL0-7 in BC, the expression of CARLo-7 in BC and adjacent normal tissues was evaluated by qRT-PCR. As shown in Figure 1A, CARLo-7 levels were significantly upregulated in BC tissues compared with paired adjacent normal tissues, corresponding with the earlier study. Moreover, we further analyzed the clinicopathological characteristics of BC patients and found that high CARLo-7 expression in BC tissues was closely associated with higher histological grade and clinical stage and lymph nodes metastasis (Table 1). T24 and HT1197 are frequently used cell models of bladder cancer, and our pre-test results indicated that silencing CARLo-7 suppressed cell proliferation of these cells more efficiently than other bladder cancer cell lines. Thus BC cell lines T24 and HT1197 were selected to elucidate the mechanisms of the CARLo-7 in BC initiation and progression in our study. SV-HUC-1, a human epithelial SV40 immortalized uroepithelium cell line, was used as a control. The RT-PCR result showed that CARLo-7 was overexpressed in T24 and HT1197cells compared with SV-HUC-1 (Figure 1B). Collectively, our results showed that lncRNA CARLo-7 was upregulated and correlated with poor prognosis in BC patients.
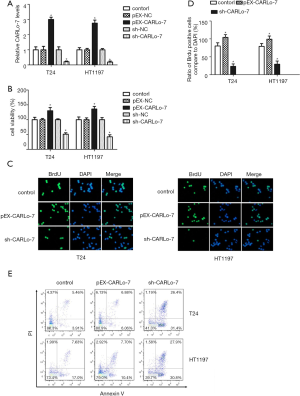
Enforced CARLo-7 expression promoted proliferation while silencing CARLo-7 suppressed proliferation and induced apoptosis of BC cells
To explore the biological function of CARLo-7 in BC cells, we enforced expression or knocked down CARLo-7 expression in T24 and HT1197 cells by transfecting these cells with pEX-CARLo-7, pEX-NC, sh-CARLo-7, or sh-NC vector. As shown in Figure 2A, CARLo-7 was overexpressed in T24 and HT1197 cells transfected with pEX-CARLo-7 compared with cells transfected with pEX-NC (P<0.05). Moreover, CARLo-7 expression was dramatically reduced in T24 and HT1197 cells transfected with sh-CARLo-7 compared with cells transfected with sh-NC (P<0.05). The influence of CARLo-7 on cell proliferation of T24 and HT1197 cells was evaluated by cell viability assay. As shown in Figure 2B, enforced CARLo-7 expression significantly increased the cell viability of T24 and HT1197 cells compared with cells transfected with pEX-NC (P<0.05), while silencing CARLo-7 decreased the cell viability of T24 and HT1197 cells compared with cells transfected with sh-NC (P<0.05). These results showed that enforced CARLo-7 expression promoted cell proliferation of BC cells while silencing CARLo-7 suppressed proliferation. To further confirm this, the BrdU assay was conducted to evaluate cell proliferation in T24 and HT1197 cells with CARLo-7 overexpression or knockdown. As shown in Figure 2C,D, the percentage of BrdU positive cells was increased dramatically in the T24 and HT1197 cells transfected with pEX-CARLo-7, while silencing CARLo-7 decreased the percentage of BrdU positive cells in T24 and HT1197 cells, indicating that CARLo-7 overexpression facilitated proliferation while silencing CARLo-7 suppressed proliferation of T24 and HT1197 cells. T24 and HT1197 cells were transfected with pEX-CARLo-7 or sh-CARLo-7; cell apoptosis was evaluated by flow cytometry to evaluate the influence of CARLo-7 overexpression or knockdown on apoptosis. As shown in Figure 2E, CARLo-7 overexpression had no apparent influence on cell apoptosis of T24 and HT1197 cells (P>0.05). On the contrary, silencing CARLo-7 increased the percentage of Annexin V and PI double-positive cells in T24 and HT1197 significantly, showing that CARLo-7 knockdown induced apoptosis. These results show that CARLo-7 overexpression promoted the proliferation of T24 and HT1197 cells but did not affect cell apoptosis while silencing CARLo-7 inhibited proliferation and induced apoptosis of T24 and HT1197 cells.
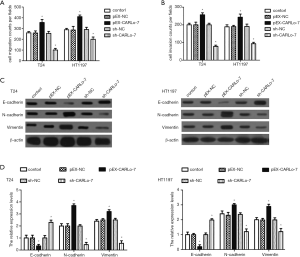
Enforced CARLo-7 expression facilitated migration, invasion, and EMT of BC cells while silencing CARLo-7 had the contrary effects
T24 and HT1197 cells transfected with pEX-CARLo-7 or sh-CARLo-7 expression vector were used for Transwell cell migration and invasion assay to evaluate the influence of CARLo-7 on cell migration and invasion. As shown in Figure 3A,B, enforced CARLo-7 expression promoted cell migration and invasion of T24 and HT1197 cells, while silencing CARLo-7 had the contrary effects. Furthermore, the protein expression of EMT markers, including E-cadherin, N-cadherin, and Vimentin, was analyzed by western blot. We found CARLo-7 overexpression suppressed E-cadherin expression and increased N-cadherin and Vimentin expression in T24 and HT1197 cells, while CARLo-7 knockdown decreased the protein expression of N-cadherin and vimentin and increased E-cadherin expression (Figure 3C,D). These results showed that enforced CARLo-7 expression promoted migration, invasion, and EMT of T24 and HT1197 cells, while silencing CARLo-7 had the contrary effects.
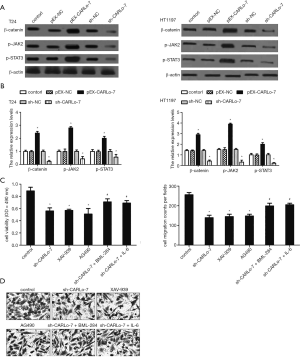
Wnt/β-catenin and JAK2/STAT3 signaling pathways participated in the regulation of cell proliferation and migration by CARLo-7
Accumulated studies demonstrate that Wnt/β-catenin and JAK2/STAT3 signaling pathways involve migration, invasion, and EMT of BC cells (15,16,18,19). To determine if Wnt/β-catenin and JAK2/STAT3 signaling pathways were involved in migration, invasion, and EMT of BC cells mediated by CARLo-7, the protein expression of β-catenin, p-JAK2, and p-STAT3 in T24 and HT1197 cells was evaluated by western blot. CARLo-7 overexpression increased the expression levels of β-catenin, p-JAK2, and p-STAT3 in T24 and HT1197 cells, as shown in Figure 4A,B. Simultaneously, silencing CARLo-7 decreased β-catenin, p-JAK2, and p-STAT3 in T24 and HT1197 cells. We further used XAV-939 (inhibitor of Wnt/β-catenin signaling) and AG490 (inhibitor of JAK2/STAT3 signaling) to determine whether Wnt/β-catenin and JAK2/STAT3 regulate the cell proliferation and migration of BC cells. Our results showed that XAV-939 and AG490 suppressed cell proliferation and migration of T24 cells, like CARLo-7 knockdown (Figure 4C,D). On the contrary, activation of Wnt/β-catenin signaling by BML-284, or JAK2/STAT3 pathway by IL-6, abolished the effects on cell proliferation and migration caused by CARLo-7 knockdown (Figure 4C,D). These results showed that Wnt/β-catenin and JAK2/STAT3 signaling pathways were involved in regulating cell proliferation and migration by CARLo-7 in BC cells.
Discussion
Though NMIBC had a favorable prognosis with a low tendency to metastasis compared with MIBC, it has a high rate of recurrence despite surgery resection and local chemotherapy (20). Furthermore, the more aggressive MIBC still has a dismal prognosis despite the recent advance in cancer therapy (21). Thus, it is necessary to find new biomarkers to predict the prognosis of BC patients. In this study, we found that CARLo-7 was significantly upregulated in both BC tissues and cell lines, showing that CARLo-7 might involve in the progression of BC. Thus, we evaluated the correlations between CARLo-7 expression and clinicopathologic features of BC patients and found that high CARLo-7 expression was closely associated with advanced histological grade, lymph nodes metastasis, and higher clinical stage. These results suggested that CARLo-7 could be a biomarker for predicting the prognosis of BC. Also, our results showed that cell proliferation, migration, invasion, and EMT were all significantly suppressed by CARLo-7 knockdown in BC cells. However, enforced CARLo-7 expression facilitated proliferation, migration, invasion, and EMT of BC cells. Therefore, these results further confirmed that CARLo-7 could be an effective biomarker for BC prognosis.
There are increasing evidence lncRNAs participate in the initiation, progression, EMT, and metastasis of BC. For example, the upregulation of H19 and MALAT-1 increased the metastasis property of BC cells (22,23). Moreover, high expression of UCA1 was demonstrated to facilitate proliferation, tumorigenesis, and chemoresistance of BC cells through regulating the Wnt/β-catenin pathway (24). TGFβ1 induced EMT of BC cells, and this was partially mediated by lncRNA ZEB2NAT through increasing ZEB2 expression (25). Our results and a previous study (9) indicated CARLo-7 could be a biomarker for BC prognosis, but the exact role of CARLo-7 in BC patients has not been elucidated. In our study, we found that CARLo-7 was highly expressed in BC tissues. Thus, we hypothesized that CARLo-7 might promote the progression of BC. We knocked down CARLo-7 in T24 and HT1197 cells by transfecting with sh-CARLo-7 and found that silencing CARLo-7 suppressed proliferation, migration, invasion, and induced apoptosis BC cells significantly. More importantly, we further detected the expression levels of EMT-related nj found that CARLo-7 knockdown suppressed EMT process. Taken together, our study firstly revealed CARLo-7 might function as an oncogene and play a role in the progression of BC.
Wnt/β-catenin pathway is crucial for embryo development and tissue homeostasis. Also, Wnt/β-catenin signaling pathway is demonstrated to be involved in tumor initiation, progression, and metastasis of various cancers, including breast cancer (26), glioblastoma (27), esophageal cancer (28), and ovarian cancer (29). JAK/STAT signaling pathway consists of three main components: cell surface receptor, JAKs, and two STAT proteins (30). Dysregulation of the JAK/STAT pathway also plays a role in the initiation and progression of BC (20,31). In our study, we found that XAV-939 and AG490 both suppressed cell proliferation and migration of BC cells, which suggested that Wnt/β-catenin and JAK2/STAT3 pathway were activated in BC. Moreover, silencing CARLo-7 inhibited the activation of the JAK2/STAT3 and Wnt/β-catenin pathway significantly. Activating the JAK2/STAT3 and Wnt/β-catenin pathway by BML-284 or IL-6 significantly reversed the inhibition effects of CARLo-7 knockdown on cell proliferation and migration. Therefore, our results suggested that CARLo-7 exerted an oncogenic role in BC cells by activating JAK2/STAT3 and Wnt/β-catenin signaling pathways.
Conclusions
In summary, our results showed lncRNA CARLo-7 functioned as an oncogene in BC cells. Enforced CARLo-7 expression facilitated proliferation, migration, invasion, and EMT of BC cells, while silencing CARLo-7 had the contrary effects. Moreover, silencing CARLo-7 inhibited the activation of the JAK2/STAT3 and Wnt/β-catenin pathway significantly. Our study supplied new insights for the pathogenesis of BC and might supply new therapeutic targets for improving BC treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1293
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1293
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1293). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee approved this study of Qianfoshan Hospital Affiliated to Shandong University, SYXK (Jing) 2017-0015. Written informed consent was obtained from all patients enrolled in this study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Zhang W, Wang R, Ma W, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med 2019;7:431. [Crossref] [PubMed]
- Smolensky D, Rathore K, Cekanova M. Molecular targets in urothelial cancer: detection, treatment, and animal models of bladder cancer. Drug Des Devel Ther 2016;10:3305-22. [Crossref] [PubMed]
- Tan Y, Zhang T, Liang C. Circular RNA SMARCA5 is overexpressed and promotes cell proliferation, migration as well as invasion while inhibits cell apoptosis in bladder cancer. Transl Cancer Res 2019;8:1663-71. [Crossref]
- Knollman H, Godwin JL, Jain R, et al. Muscle-invasive urothelial bladder cancer: an update on systemic therapy. Ther Adv Urol 2015;7:312-30. [Crossref] [PubMed]
- Vartolomei MD, Ferro M, Cantiello F, et al. Validation of Neutrophil-to-lymphocyte Ratio in a Multi-institutional Cohort of Patients With T1G3 Non-muscle-invasive Bladder Cancer. Clin Genitourin Cancer 2018;16:445-52. [Crossref] [PubMed]
- Ferro M, Vartolomei MD, Russo GI, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol 2019;37:507-14. [Crossref] [PubMed]
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43:904-14. [Crossref] [PubMed]
- Amir H, Khan MA, Feroz S, et al. CARLo-7-A plausible biomarker for bladder cancer. Int J Exp Pathol 2019;100:25-31. [Crossref] [PubMed]
- Zhang L, Kang W, Lu X, et al. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. 2018;17:1886-900.
- Cui Y, Shen G, Zhou D, et al. CASC11 Overexpression Predicts Poor Prognosis and Regulates Cell Proliferation and Apoptosis in Ovarian Carcinoma. Cancer Manag Res 2020;12:523-9. [Crossref] [PubMed]
- Tong W, Han TC, Wang W, et al. LncRNA CASC11 promotes the development of lung cancer through targeting microRNA-302/CDK1 axis. Eur Rev Med Pharmacol Sci 2019;23:6539-47. [PubMed]
- Kajino T, Shimamura T, Gong S, et al. Divergent lncRNA MYMLR regulates MYC by eliciting DNA looping and promoter-enhancer interaction. EMBO J 2019;38:e98441. [Crossref] [PubMed]
- Garg M, Maurya N. WNT/beta-catenin signaling in urothelial carcinoma of bladder. World J Nephrol 2019;8:83-94. [Crossref] [PubMed]
- Yang C, Zhang W, Wang L, et al. Musashi-2 promotes migration and invasion in bladder cancer via activation of the JAK2/STAT3 pathway. Lab Invest 2016;96:950-8. [Crossref] [PubMed]
- Chen Z, Du Y, Liu X, et al. EZH2 inhibition suppresses bladder cancer cell growth and metastasis via the JAK2/STAT3 signaling pathway. Oncol Lett 2019;18:907-15. [Crossref] [PubMed]
- Zhang Z, Zhou C, Chang Y, et al. Long non-coding RNA CASC11 interacts with hnRNP-K and activates the WNT/beta-catenin pathway to promote growth and metastasis in colorectal cancer. Cancer Lett 2016;376:62-73. [Crossref] [PubMed]
- Guo J, Chen Z, Jiang H, et al. The lncRNA DLX6-AS1 promoted cell proliferation, invasion, migration and epithelial-to-mesenchymal transition in bladder cancer via modulating Wnt/beta-catenin signaling pathway. Cancer Cell Int 2019;19:312. [Crossref] [PubMed]
- Zhou Q, Chen S, Lu M, et al. EFEMP2 suppresses epithelial-mesenchymal transition via Wnt/beta-catenin signaling pathway in human bladder cancer. Int J Biol Sci 2019;15:2139-55. [Crossref] [PubMed]
- Jiang F, Qi W, Wang Y, et al. lncRNA PEG10 promotes cell survival, invasion and migration by sponging miR-134 in human bladder cancer. Biomed Pharmacother 2019;114:108814. [Crossref] [PubMed]
- Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639-53. [Crossref] [PubMed]
- Luo M, Li Z, Wang W, et al. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett 2013;333:213-21. [Crossref] [PubMed]
- Ying L, Chen Q, Wang Y, et al. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst 2012;8:2289-94. [Crossref] [PubMed]
- Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J 2014;281:1750-8. [Crossref] [PubMed]
- Zhuang J, Lu Q, Shen B, et al. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep 2015;5:11924. [Crossref] [PubMed]
- Teufel S, Hartmann C. Wnt-signaling in skeletal development. Curr Top Dev Biol 2019;133:235-79. [Crossref] [PubMed]
- Chen Q, Cai J, Wang Q, et al. Long Noncoding RNA NEAT1, Regulated by the EGFR Pathway, Contributes to Glioblastoma Progression Through the WNT/beta-Catenin Pathway by Scaffolding EZH2. Clin Cancer Res 2018;24:684-95. [Crossref] [PubMed]
- Zhu L, Zhang X, Fu X, et al. TIPE2 suppresses progression and tumorigenesis of esophageal carcinoma via inhibition of the Wnt/beta-catenin pathway. J Transl Med 2018;16:7. [Crossref] [PubMed]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013;13:11-26. [Crossref] [PubMed]
- Aaronson DS, Horvath CM. A road map for those who don't know JAK- STAT. Science 2002;296:1653-5. [PubMed]
- Li C, Cao Y, Zhang L, et al. LncRNA IGFBP4-1 promotes tumor development by activating Janus kinase-signal transducer and activator of transcription pathway in bladder urothelial carcinoma. Int J Biol Sci 2020;16:2271-82. [Crossref] [PubMed]
(English language editor: J. Chapnick)


