
Efficacy and safety of keishibukuryogan, a traditional Japanese Kampo medicine, for hot flashes in prostate cancer patients receiving androgen deprivation therapy
Introduction
The gold standard for the management of prostate cancer (PC) is hormonal therapy, such as androgen deprivation therapy (ADT), along with radical prostatectomy and radiotherapy. Especially in Japan, ADT is widely used as a primary therapy for patients with advanced PC, as a neoadjuvant/adjuvant therapy combined with various radiotherapies (1). On the other hand, numerous adverse effects such as erectile dysfunction, low libido, menopausal symptoms, and metabolic syndrome occur frequently with ADT (2). Among these adverse events, hot flashes are the most common and result in decreased patient quality of life (QOL) (3). Although some drugs have been reported to be effective for hot flashes (4,5), there are currently no established treatments.
Keishibukuryogan (Gyejibokryeong-hwan in Korea and Guizhi-Fuling-Wan in China) is a traditional Japanese herbal formula (Kampo) consisting of five medicinal herbs: Cinnamomi cortex, Hoelen, Paeoniae Radix, Moutan cortex, and Persicae semen. It is indicated for female menopausal symptoms including headache, dizziness, hot flashes, and stiff shoulders (6). In Japan, keishibukuryogan is often prescribed to improve hot flashes associated with ADT in PC patients. However, there is lack of data on the efficacy and safety of keishibukuryogan for hot flashes. Hence, the present study investigated the efficacy and safety of keishibukuryogan for hot flashes in PC patients receiving ADT and evaluated the predictive factors for response to keishibukuryogan treatment.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-901).
Methods
Study design
This study was approved by the Ethical Committee of the Kanazawa University Graduate School of Medical Science (No. 2017-214) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients enrolled completed the informed consent form. Tsumura keishibukuryogan extract granules for ethical use (TJ-25; Tsumura & Co., Tokyo, Japan) were used for this study. This prospective single-arm cross-sectional study evaluated the efficacy and safety of TJ-25 for hot flashes among PC patients receiving ADT (UMIN 000031606). The primary endpoint was the efficacy of TJ-25 in improving the strength, frequency, and duration of hot flashes. Included in the secondary endpoints were its efficacy in ameliorating menopausal symptoms and its safety, including changes in total testosterone (TT) and prostate specific antigen (PSA) levels.
Study population
Factors determining eligibility in PC patients receiving ADT at Kanazawa University Hospital who had hot flashes included: desire for treatment, performance status of 0–2, a prognosis of one year or more, and the ability to take the drug orally. We excluded patients with severe mental illness; serious liver or renal dysfunction (>3-fold the upper limit of normal AST and ALT values and >2-fold creatinine value); any current use of other herbal medicine and/or selective serotonin reuptake inhibitors and/or serotonin-noradrenalin reuptake inhibitors; previous use of TJ-25 within the 2 weeks prior; a history of allergies to any herbal medicines; castration-resistant PC; positive for Human Immunodeficiency Virus, Hepatitis B Virus, or Hepatitis C Virus; and others, including patients who were judged by the investigator as ineligible. Finally, 30 patients were enrolled in the present study between April 2018 and October 2018.
Study protocol
After providing written informed consent, all patients underwent screening tests at a baseline visit, including assessments of their current medications and medical illnesses, physical examinations, and blood biochemical testing. Patient characteristics, including age, body weight, body mass index (BMI), PC status (clinical stage, Gleason score, and duration of cancer), past history of radiation or radical prostatectomy, details of ADT (luteinizing hormone-releasing hormone (LH-RH) agonist/antagonist), and current complications, were recorded.
Eligible patients were administrated TJ-25 at a dose of 2.5 g three times daily for 12 weeks. Medical interviews, blood tests, and checks for any adverse effects were performed 4, 8, and 12 weeks after treatment for all participants. During the trial, all patients were asked to keep a daily describing their hot flashes. The diary included information regarding the strength, frequency, and mean duration of hot flashes. The hot flash strength was assessed by the visual analog scale (VAS), and duration was described in three categories: <3, ≥3 min and <5, or ≥5 min. The diary was collected by the attending doctors at each visit. In addition, a questionnaire evaluating the aging male symptoms (AMS) scale was administered to evaluate menopausal symptoms at the baseline, 4-, 8-, 12-week visits. Blood biochemical analyses including liver function (AST, ALT, ALP, γ-GTP, and T-Bil values), renal function (Cr and BUN values), and PSA levels were also performed at every visit. TT levels were measured at the baseline and 12-week visits. Manuals on consent and research procedure were standardized to address potential sources of bias.
Laboratory assays
All blood samples were collected between 09:00 and 11:00 at each visit. Blood biochemical data, PSA, and TT were measured promptly, using the commercially available routine autoanalyzer in our hospital. The serum PSA levels were measured by EIA with a minimum detectable concentration of 0.008 ng/mL, and TT levels were measured by electrochemiluminescence immunoassay (CobasTM system, Roche Diagnostic, Co., Tokyo, Japan).
Sample size
This was a prospective observational pilot study. The sample size was calculated based on the number of patients undergoing hormone therapy per year at research facilities and incidence rate of hot flashes, which was set as the number of cases considered to be practicable in daily medical practice within the registration period.
Statistical analysis
The strength of hot flashes was judged quantitatively with a 100 mm ruler of VAS. The strength and frequency of hot flashes were evaluated as the mean value recorded in a diary for one week prior to each visit. The duration of hot flashes was assessed one day before each visit. Changes in the strength and frequency of hot flashes from baseline to each visit were compared using paired t-tests, and changes in duration were compared by the Wilcoxon’s signed rank test. Comparisons in the scores of the AMS scale and each blood laboratory test between baseline and each visit were performed using the Wilcoxon’s signed rank test.
Next, responder analysis was conducted to identify the predictive factors for response to TJ-25 treatment for hot flashes. All patients were divided into two or three groups according to their median age, body weight, BMI, and duration of PC, as well as the presence of radical prostatectomy, radiation, and complications. They were also classified as follows: clinical stage T1/T2 or T3/T4; Gleason score <8, 8, or ≥9; and LH-RH agonist or antagonist. Changes from the baseline to 12-week visits in hot flashes symptoms were compared by unpaired t-test. Missing data items repeatedly measured over time were not complemented.
In all analyses, P<0.05 was considered statistically significant. All statistical analyses were commissioned to I’CROS JAPAN Co., Ltd. (Tokyo, Japan).
Results
Participant characteristics
A total 30 patients were recruited for the present study, and all met the inclusion criteria and were eligible for this trial. The patients’ characteristics are summarized in Table 1. Their age [mean ± standard deviation (SD)] was 69.5±6.0, and BMI was 24.0±3.7. The duration of PC was 3.1±4.2 years. Eleven (36.7%) and seven (23.3%) patients had received radiotherapy and radical prostatectomy, respectively.
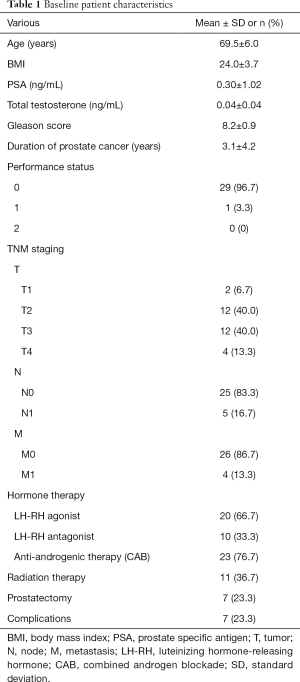
Full table
Finally, 25 of 30 participants completed the TJ-25 treatment for 12 weeks (Figure 1). Five patients discontinued treatment due to withdrawal of consents (three cases), adverse effects (one case of sleep disorder), and investigator decisions (one case).
Primary endpoint
The strength of the hot flashes was significantly improved at the 4-week visit, and its improvement lasted for 12 weeks after the treatment (Figure 2A). The frequency significantly decreased by the 8-week visit and showed a slight, non-significant improvement at the 12-week visit (Figure 2B). A reduction in the duration of hot flashes was observed beginning four weeks after treatment and was significant at the 8- and 12-week visits (Figure 2C).
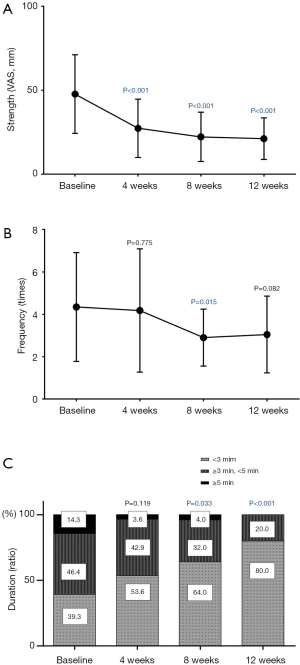
Secondary endpoints
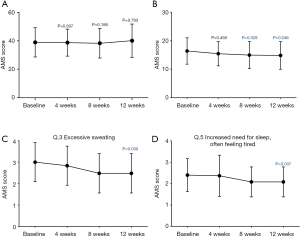
The total score of the AMS scale was not significantly changed by the 12-week treatment (Figure 3A). On the other hand, scores of the somatic subscale were significantly improved at the 8- and 12-week visits, although no significant changes were observed in scores of the psychological and sexual sub-scales at each visit (Figure 3B). Of the scores of the somatic subscale, the patients had significant improvements in questions 3 (excessive sweating) and 5 (increased need for sleep) (Figure 3C,D).
No biochemical parameters, including PSA and TT levels, showed significant changes during this trial. Six adverse events—increased GOT value, nausea, general malaise, thirst, reduced appetite, and sleep disorder—were observed in five cases, but all were mild. Two cases had adverse events that were undeniably associated with TJ-25 administration: nausea and general malaise in one case and sleep disorder in another. Treatment was discontinued in only one case of an adverse effect (sleep disorder).
Responder analysis
TJ-25 administration became increasingly effective in patients with obesity, a medical history of radiation, and a longer duration of PC (Table 2). The frequency of hot flashes significantly improved in patients with a BMI higher than the median value of 24.2 (Figure 4). Hot flash strength was significantly decreased in patients with a history of radiation and a PC duration longer than 1.58 years. No predictive factors for improving the duration of hot flashes were determined.

Full table

Discussion
Keishibukuryogan (i.e., Gyejibokryeong-hwan or Guizhi-Fuling-Wan) has been widely used for the treatment of female climacteric syndrome in China, Korea, and Japan (6-8). This Kampo is effective for various symptoms caused by blood stasis, such as hot flashes, muscle stiffness, dizziness, and headache (9,10). Especially in Japan, Kampo treatments, which are covered by the Japanese national health insurance system, are very common in daily clinical practice in men and women.
Recently, it has been widely accepted that elderly men also suffer from various menopausal symptoms due to the decline in testosterone associated with aging (11,12). Previous studies have demonstrated the efficacy of Kampo treatments, including TJ-25, for hypogonadal men (13,14). ADT for PC patients often causes various menopausal symptoms including hot flashes, fatigue, and depression due to excessive testosterone decline (3). Hot flashes are one of the most common adverse effects of ADT and lead to decreased patient QOL. Therefore, many clinicians must treat hot flashes in patients receiving ADT.
There are various hypotheses regarding the mechanisms for the development of hot flashes with ADT. Disorders of the hypothalamic thermoregulatory center may be one possible mechanism, and sex hormone levels have potential effects on noradrenaline and endorphins in the hypothalamus, which are likely to be hypothalamic neurotransmitters (15,16). Another report suggested that serotonin may play an important role in the occurrence of hot flashes (17). Alternatively, calcitonin gene related peptide (CGRP), which is one of the vasodilator neuropeptides, is reportedly closely associated with hot flashes (18). In this report, CGRP administration resulted in elevated skin temperature in castrated rats, which was suppressed by 17-beta-estradiol and progesterone (18). However, there is currently no established mechanism for the development of hot flashes with ADT.
In the present study, TJ-25 administration improved the strength, frequency, and duration of hot flashes. The strength of hot flashes was significantly improved at the 4-, 8-, and 12-week visits, and frequency was significantly decreased at the 8-week visit. In addition, a reduction in hot flash duration was observed 8 and 12 weeks after treatment. This is the first prospective study to demonstrate efficacy of TJ-25 for hot flashes among PC patients receiving ADT.
Some mechanisms of the efficacy of TJ-25 for hot flashes have been proposed in ovariectomized rat models and postmenopausal women. An experimental study demonstrated that TJ-25, as well as 17-beta estradiol, inhibited the synthesis and release of CGRP, resulting in suppression of CGRP-induced hot flashes in the ovariectomized rat (19,20). In addition, TJ-25 improved hot flashes by reducing serum interleukin (IL)-8 and circulating monocyte chemotactic protein-1, which are involved in vascular inflammation and vasodilation, in postmenopausal women with hot flashes (21). On the other hand, few data on the efficacy of TJ-25 administration in men are currently available. One previous study revealed an increase in CGPR levels among PC patients with castration or LH-RH agonist injection, suggesting a similar mechanism for the occurrence of hot flashes in women and men (22).
We found that TJ-25 was more effective in patients with obesity, a medical history of radiation, or a longer duration of PC, and reasons for this are unclear. Since the efficacy and adverse effects of the same Kampo medicine are not identical for each patient, this is selected according to “Sho” (pattern of symptoms, excess or insufficient symptom-complex) of the patient. TJ-25 is generally recommended to patients displaying the excess (Jitsu-Sho) condition. Although we did not judge the “Sho” of each patient in the present study, many patients with these predictive factors were likely to display the excess condition. Alternatively, serum levels of inflammatory cytokines or various hormones significantly associated with hot flashes were likely to differ between the patients with these predictive factors and those without. Evaluating changes in the serum levels of various hormones and inflammatory cytokines due to TJ-25 administration warrants further investigation.
In the present study, although three adverse events, which were undeniably associated with TJ-25 administration, were observed in two cases, all could be tolerated. PSA and TT levels were not significantly changed by TJ-25 administration, suggesting that TJ-25 had no impacts on cancer control.
There were some limitations in the present study. Firstly, this study comprised a small number of subjects. In addition, this is a single-arm cross-sectional study and not a randomized controlled trial, and then the present results were likely to be affected by recall bias. Therefore, the efficacy of TJ-25 may include some placebo effects. Moreover, eligible patients visited our hospital every 4 weeks during the trial. It is possible that frequent contact with the doctors and coordinators had a positive impact on patients’ mental condition and anxiety. Therefore, further prospective randomized controlled trials including large numbers of participants are certainly required to validate the results of this study.
In conclusion, Keishibukuryogan is an effective and safe treatment for hot flashes in PC patients receiving ADT.
Acknowledgments
The authors want to highlight the significant technical contributions by Toshitaka Kido and Keita Mizuno from Tsumura & Company, Tokyo, Japan. The study team deeply appreciates the technical support in data collections by some coordinators in Innovative Clinical Research Center, Kanazawa University.
Funding: This work was supported in specific funding from Tsumura & Co., Tokyo, Japan.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-901
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-901
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-901
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-901). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethical Committee of the Kanazawa University Graduate School of Medical Science (2017-214) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Witten informed consent was obtained from all study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Namiki M, Ueno S, Kitagawa Y. Role of hormonal therapy for prostate cancer: perspective from Japanese experiences. Transl Androl Urol 2012;1:160-72. [PubMed]
- Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825-36. [Crossref] [PubMed]
- Nishiyama T, Kanazawa S, Watanabe R, et al. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol 2004;11:735-41. [Crossref] [PubMed]
- Irani J, Salomon L, Oba R, et al. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin releasing hormone analogues for prostate cancer: a double-blind, randomised trial. Lancet Oncol 2010;11:147-54. [Crossref] [PubMed]
- Moraska AR, Atherton PJ, Szydlo DW, et al. Gabapentin for the management of hot flashes in prostate cancer survivors: a longitudinal continuation Study-NCCTG Trial N00CB. J Support Oncol 2010;8:128-32. [PubMed]
- Namiki T, Sato H, Matsumoto Y, et al. Identification of a predictive biomarker for the beneficial effect of keishibukuryogan, a Kampo (Japanese traditional) medicine, on patients with climacteric syndrome. Evid Based Complement Alternat Med 2014;2014:962109. [Crossref] [PubMed]
- Park JM, Yang JM, Kim DI. A clinical trial to verify the quality of life improvement efficacy of Dangguijakyak-san and Gyejibongnyeong-hwan granulation in postmenopausal women. J Orient Obstet Gynecol 2007;20:213-28.
- Ushiroyama T, Ikeda A, Sakuma K, et al. Comparing the effects of estrogen and an herbal medicine on peripheral blood flow in post-menopausal women with hot flashes: hormone replacement therapy and gui-zhi-fu-ling-wan, a Kampo medicine. Am J Chin Med 2005;33:259-67. [Crossref] [PubMed]
- Terauchi M, Hiramitsu S, Akiyoshi M, et al. Effects of three Kampo formulae: Tokishakuyakusan (TJ-23), Kamishoyosan (TJ-24), and Keishibukuryogan (TJ-25) on Japanese peri- and postmenopausal women with sleep disturbances. Arch Gynecol Obstet 2011;284:913-21. [Crossref] [PubMed]
- Park JS, Park S, Cheon CH, et al. Effects and safety of gyejibongnyeong-hwan on dysmenorrhea caused by blood stagnation: a randomized controlled trial. Evid Based Complement Alternat Med 2013;2013:424730. [Crossref] [PubMed]
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5-15. [Crossref] [PubMed]
- Lunenfeld B, Arver S, Moncada I, et al. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male 2012;15:187-97. [Crossref] [PubMed]
- Amano T, Imao T, Takemae K. Clinical efficacy of Japanese traditional herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male 2010;13:166-73. [Crossref] [PubMed]
- Tsujimura A, Nonomura N. Recent topics related to testosterone deficiency syndrome in Japan. Asian J Androl 2011;13:558-62. [Crossref] [PubMed]
- Kouriefs C, Georgiou M, Ravi R. Hot flushes and prostate cancer: pathogenesis and treatment. BJU Int 2002;89:379-83. [Crossref] [PubMed]
- Dyer RG, Grossman R. Opioid modulation of the response of preoptic neurones to stimulation of the ventral noradrenergic tract in female rats. J Physiol 1988;400:631-44. [Crossref] [PubMed]
- Berendsen HH. The role of serotonin in hot flushes. Maturitas 2000;36:155-64. [Crossref] [PubMed]
- Yuzurihara M, Ikarashi Y, Noguchi M, et al. Prevention by 17beta-estradiol and Progesterone of Calcitonin Gene-Related Peptide-Induced Elevation of Skin Temperature in Castrated Male Rats. Urology 2004;64:1042-7. [Crossref] [PubMed]
- Noguchi M, Ikarashi Y, Yuzurihara M, et al. Up-regulation of calcitonin gene-related peptide receptors underlying elevation of skin temperature in ovariectomized rats. J Endocrinol 2002;175:177-83. [Crossref] [PubMed]
- Noguchi M, Ikarashi Y, Yuzurihara M, et al. Effects of 17-β-estradiol and the Japanese herbal medicine Keishi bukuryo-gan on the release and synthesis of calcitonin gene-related peptide in ovariectomized rats. J Pharmacol Sci 2003;93:80-6. [Crossref] [PubMed]
- Yasui T, Matsui S, Yamamoto S, et al. Effects of Japanese traditional medicines on circulating cytokine levels in women with hot flashes. Menopause 2011;18:85-92. [Crossref] [PubMed]
- Spetz AC, Pettersson B, Varenhorst E, et al. Momentary increase in plasma calcitonin gene-related peptide is involved in hot flashes in men treated with castration for carcinoma of the prostate. J Urol 2001;166:1720-3. [Crossref] [PubMed]



