
Transverse testicular ectopia associated with persistent Mullerian duct syndrome in infertile male: two case reports and literature review
Introduction
Transverse testicular ectopia (TTE) is a rare form of testicular ectopia, in which both testes descend through the same inguinal canal (1). Persistent Mullerian duct syndrome (PMDS) is characterized by the persistence of Mullerian duct structures in males with normal karyotype. TTE associated with PMDS occurs in approximately 20% of all the TTE cases (2). Most cases of TTE associated with PMDS were found during repair of inguinal hernia or cryptorchidism surgery in children. Compared with children, adult cases are even more peculiar and challenging. First, adult patients usually have a more complex medical history or surgical history, making the diagnosis and treatment trickier. Second, evaluation and examination of the adult patients should be more comprehensive, much attention should be paid to their fertility, potential malignancy and mental health. Also, surgical management of adult patients is complex. The location and degeneration degree of testis, the length of spermatic cord, the size and location of Mullerian duct all affect the operative choice, and should all be taken into careful consideration. Herein, we report two cases of TTE associated with PMDS in two adult patients. The clinical characteristics, pathogenesis and management of this disease are also reviewed. We present the following cases in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-888).
Case presentation
Case report 1
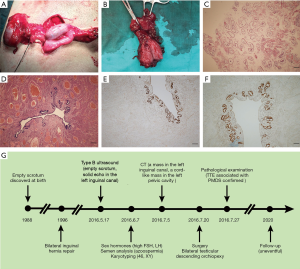
A 28-year-old man presented with bilateral empty scrotum, who underwent bilateral oblique inguinal hernia repair 20 years ago in a rural hospital. On physical examination, no other abnormalities were found except for bilateral impalpable testes. Type B ultrasound revealed a solid echo about 2.1 cm × 1.9 cm in size inside the left inguinal canal. The scrotum and the right inguinal canal showed no testis image. Computed tomography (CT) scan revealed an oval low-density mass in the left inguinal canal and a cord-like low-density mass in the left pelvic cavity. No sperm was found in the semen. The karyotype was 46, XY. Sex hormone showed follicle-stimulating hormone (FSH) 29.18 (1.5–12.5) mIU/mL, luteinizing hormone (LH) 11.5 (1.7–8.6) mIU/mL, prolactin (PRL) 41.39 (4.79–23.3) ng/mL, E2 17.03 (25.8–60.7) pg/mL, and T 5.37 (2.8–8.8) ng/mL. Bilateral testicular descending orchiopexy with transperitoneal laparoscopy was undertaken. During the operation, the right testis was found located in the left abdominal cavity, and the epididymis and vas deferens were detached from the testis. The left testis was in the left pelvic cavity with normal epididymis and vas deferens (Figure 1A). A tubular structure adhering to the left spermatic cord around 1.5 cm in diameter and 5 cm in length was found and resected (Figure 1B). The two testes were brought down to the scrotum through the inguinal canal. Two patches were used to repair the deep inguinal ring. Testis biopsies showed only Sertoli cells existed in the seminiferous tubules, and Johnsen score was 2 (Figure 1C). As for the tubular structure, pathological examination showed a muscular tube lined by columnar epithelium and a thin compact layer of stroma consistent with a uterus (Figure 1D). Immunohistochemical staining confirmed the result, which presented positive staining for estrogen receptor (ER) and progesterone receptor (PR) (Figure 1E,F). Therefore, a diagnosis of TTE associated with PMDS and non-obstructive azoospermia was made. Postoperative course was uneventful. The diagnose and treatment process was illustrated in Figure 1G.
Case report 2
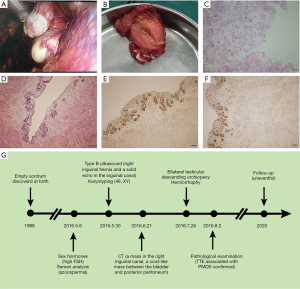
Another 28-year-old man presented with bilateral impalpable testes and empty scrotum. On physical examination, a mass about 2 cm in diameter was found in the right inguinal region. Type B ultrasound revealed right inguinal hernia and a solid echo inside the right inguinal canal, and the size was about 2.2 cm × 1.8 cm. The scrotum and the left inguinal canal showed no testis image. CT revealed an oval low-density mass in the right inguinal canal and a cord-like low-density mass between the bladder and posterior peritoneum. No sperm was found in the semen. The karyotype was 46, XY. Sex hormones showed FSH 16.52 mIU/mL, LH 7.08 mIU/mL, PRL 8.83 ng/mL, E2 5.00 pg/mL, and T 5.44 ng/mL. During the laparoscopic herniorrhaphy, no testis was found in the left abdominal cavity and left inguinal canal. Left spermatic cord traversed to the right pelvic cavity, which was tangled with the right spermatic cord. The two spermatic cords extended towards the right inguinal hernial sac, and two testes were found in the sac. The right testis was about 2.0 cm × 3.7 cm and the left testis was about 2.0 cm × 1.5 cm in size. Both epididymides were detached from the testes (Figure 2A). A cord-like structure adhering to the left spermatic cord was found between the bladder and posterior peritoneum, and was resected for further examination (Figure 2B). After extensive lysis of adhesions, two testes were brought down to the scrotum through the corresponding inguinal canal. Two patches were used to repair the deep inguinal ring. Testis biopsies showed only Sertoli cells existed in the seminiferous tubules, and Johnsen score was 2 (Figure 2C). The cord-like structure was confirmed to be uterus by H&E staining and immunohistochemical staining for ER and PR (Figure 2D,E,F). Therefore, a diagnosis of TTE associated with PMDS and non-obstructive azoospermia was made. Postoperative course was also uneventful. The diagnose and treatment process was illustrated in Figure 2G.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Male sex determination is mainly regulated by two testicular hormones. Testosterone, synthesized by Leydig cells, maintains the Wolffian ducts. While AMH secreted by immature Sertoli cells is responsible for the regression of Mullerian ducts (3). Aberrant function of the two hormones could lead to various genitourinary malformation, including TTE and PMDS.
By definition, PMDS involves patients with 46, XY karyotype and normal male external genitalia (usually associated with unilateral or bilateral cryptorchidism), in association with internal Mullerian duct structures in variable locations (scrotal, inguinal, or intra-abdominal). Depending on the location of testes, PMDS could be classified into three groups. The first group involves bilateral intra-abdominal testes in a position analogous to ovaries, which accounts for the majority of the cases (60–70%). The second group (20–30%) involves one testis found in a hernia sac or scrotum in association with a contralateral inguinal hernia. The third group (10%) involves both testes located in the same hernia, together with the fallopian tubes and uterus (4). Dysfunction of AMH, including mutations resulting in deficient AMH secretion and inactivity of the AMH receptor, accounts for about 85% of cases, and the remaining 15% cases are thought to be idiopathic (5).
As in our case, concurrence of PMDS and TTE in one patient is the rarest form of the syndrome. TTE is an extremely rare entity in which both testes migrate along the same inguinal canal towards the same hemi-scrotum, while the opposite inguinal canal and hemi-scrotum are empty. Based on the presence of various associated anomalies, TTE could be classified into three types. Type I is associated with inguinal hernia alone (40–50%); type II is associated with persistent or rudimentary Mullerian duct structures (30%); type III is associated with other anomalies, like hypospadias and seminal vesicle cysts (6). Publication of TTE associated PMDS cases is limited. Reported cases in recent 20 years were reviewed and summarized in Table 1.
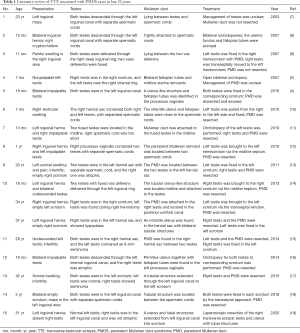
Full table
The mechanisms of TTE have not been clarified. It is likely that the mechanical effect of the persistent Mullerian duct structure produces cryptorchidism by preventing normal testicular descent. This mechanical effect may also lead to the transvers ectopia of testis across the midline.
Most patients were very young. The main clinical characteristics are unilateral or bilateral cryptorchidism and inguinal hernia. Besides, male infertility is a specific symptom for adult patients. It’s worth noting that patients with TTE are at increased risk of testicular tumor, and reports have described embryonal carcinoma, seminoma, yolk sac tumor, and teratoma. The overall incidence of malignant transformation in these testes is 18%, similar to the rate in abdominal testes. However, increased risk of testicular malignancy in TTE cannot be explained only by undescended testes. It is reported that in TTE patients, testicular tumors arose from the testis on the normal side as well as the ectopic testis, with no clear tendency in the side of tumor occurrence. Some form of tumorigenic mechanism other than cryptorchidism is expected to be involved in TTE.
TTE is sometimes diagnosed before surgery, while PMDS is usually diagnosed postoperatively with the identification of Mullerian duct structures by histological examination. For the preoperative cryptorchidism patients, extensive inquisition of medical history and physical examination are necessary. General laboratory investigations are also required, such as semen analysis, karyotype and sex hormones. Ultrasonography, CT, magnetic resonance imaging (MRI), arteriography and venography may also provide some clues.
The principles of treatment include the restoration of testes, the preservation of fertility, and the prevention of malignancy. Reduction of testes, orchidopexy and hernia repair are the common treatment procedures. The ectopic testis usually can be pulled across the median raphe and fixed in the corresponding scrotum. Biopsies of testicular tissue are highly recommended to evaluate fertility for adult patients, although all of the adult patients reported were azoospermic. In cases with a short spermatic cord or obvious testis degeneration, orchiectomy can be performed (20). Excision of the Mullerian structures is not generally recommended, as no malignancy of persistent Mullerian structures has been reported, and there is a risk of damaging the vas deferens and blood supply of testes. However, sometimes uterine hypertrophy may cause abdominal mass and discomfort, and the Mullerian ducts may hinder the reduction of testes, then excision of Mullerian remnants should be performed.
In the present cases, we successfully brought down the two testes into their corresponding scrotum through inguinal canal. During adhesiolysis, the tangled spermatic cords were carefully protected and separated, avoiding any damage to arteries and vas deferens. Testis biopsy was also performed to evaluate degeneration degree and spermatogenesis of testis. We intended to cryopreserve sperms, however, both patients were azoospermic. The Mullerian structures were resected, as they hinder the reduction of testes. After the surgery, we conducted follow-up for nearly 4 years in case of testis malignancy. Both patients show no sign of testis degeneration till now. However, they are still infertile after surgical and medication therapy due to severely impaired spermatogenesis. Also, we did not know the pathogenesis of TTE associated with PMDS in these two patients. As the diagnosis was only confirmed after pathological examination, when the patients had been discharged, they were reluctant to send blood samples for further whole exon sequencing (WES), which could help us to look for possible gene mutations. The mechanisms of TTE associated with PMDS still need further research to reveal.
Acknowledgments
Funding: The study was supported by the National Key Research and Development Program of China (2017YFC1002003), and National Natural Science Foundation of China (81871215).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-888
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-888). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gauderer MW, Grisoni ER, Stellato TA, et al. Transverse testicular ectopia. J Pediatr Surg 1982;17:43-7. [Crossref] [PubMed]
- Wuerstle M, Lesser T, Hurwitz R, et al. Persistent mullerian duct syndrome and transverse testicular ectopia: embryology, presentation, and management. J Pediatr Surg 2007;42:2116-9. [Crossref] [PubMed]
- McClelland K, Bowles J, Koopman P. Male sex determination: insights into molecular mechanisms. Asian J Androl 2012;14:164-71. [Crossref] [PubMed]
- Gokce MI, Burgu B, Aydogdu O, et al. Transverse testicular ectopia associated with persistent Mullerian duct syndrome: another entity in which magnetic resonance imaging is unreliable. Urology 2010;76:1475-7. [Crossref] [PubMed]
- Imbeaud S, Belville C, Messika-Zeitoun L, et al. A 27 base-pair deletion of the anti-mullerian type II receptor gene is the most common cause of the persistent mullerian duct syndrome. Hum Mol Genet 1996;5:1269-77. [Crossref] [PubMed]
- Hughes DT, Croitoru DP. Case report: Crossed testicular ectopia. J Pediatr Surg 2007;42:1620-2. [Crossref] [PubMed]
- Chandrasekera SK, Barber NJ, Sheriffdeen AH. Persistent Mullerian duct syndrome with transverse testicular ectopia. Urology 2003;62:1120. [Crossref] [PubMed]
- Boleken ME, Kaya M, Guran S, et al. Persistent mullerian duct syndrome with transverse testicular ectopia. Int Urol Nephrol 2007;39:1173-5. [Crossref] [PubMed]
- Marjanovic ZO, Perovic SV, Slavkovic A, et al. Transverse testicular ectopia with and without persistent Mullerian duct syndrome. Int Urol Nephrol 2007;39:1167-71. [Crossref] [PubMed]
- Chaabane W, Jarboui L, Sahnoun A, et al. Persistent Mullerian duct syndrome with torsion of a transverse testicular ectopia: first reported case. Urology 2010;76:65-6. [Crossref] [PubMed]
- Zhapa E, Castagnetti M, Alaggio R, et al. Testicular fusion in a patient with transverse testicular ectopia and persistent mullerian duct syndrome. Urology 2010;76:62-4. [Crossref] [PubMed]
- George M, Fenton E, Ferguson P. Transverse testicular ectopia with persisting mullerian remnant masquerading as right inguinal hernia and left undescended testis. ANZ J Surg 2010;80:859. [Crossref] [PubMed]
- Kaul A, Srivastava KN, Rehman SM, et al. Persistent Mullerian duct syndrome with transverse testicular ectopia presenting as an incarcerated inguinal hernia. Hernia 2011;15:701-4. [Crossref] [PubMed]
- Ju X, Li Z, Zhang C, et al. Clinical aspects and molecular genetics of persistent mullerian duct syndrome associated with transverse testicular ectopia: report of three cases. Urol Int 2013;90:83-6. [Crossref] [PubMed]
- Alp BF, Demirer Z, Guragac A, et al. Persistent Mullerian duct syndrome with transverse testicular ectopia and seminoma. Int Urol Nephrol 2014;46:1557-62. [Crossref] [PubMed]
- Telli O, Gokce MI, Haciyev P, et al. Transverse testicular ectopia: a rare presentation with persistent Mullerian duct syndrome. J Clin Res Pediatr Endocrinol 2014;6:180-2. [Crossref] [PubMed]
- Yamada K, Takahata A, Ichijo Y, et al. A case of testicular seminoma in persistent Mullerian duct syndrome with transverse testicular ectopia. Abdom Imaging 2015;40:475-9. [Crossref] [PubMed]
- Chung HS, Kim SO, Yu HS, et al. Transverse testicular ectopia associated with persistent Mullerian duct syndrome treated by transseptal orchiopexy: A case report. Medicine (Baltimore) 2018;97:e13305. [Crossref] [PubMed]
- Yu J, Wang L, Ma S, et al. Detection and Treatment of Persistent Mullerian Duct Syndrome With Transverse Testicular Ectopia. Urology 2020;140:e4-5. [Crossref] [PubMed]
- Saleem M, Ather U, Mirza B, et al. Persistent mullerian duct syndrome: A 24-year experience. J Pediatr Surg 2016;51:1721-4. [Crossref] [PubMed]


