The contemporary management of urethral strictures in men resulting from lichen sclerosus
Lichen sclerosus (LS) is an inflammatory disease that can affect both men and women and shows a predilection for the anogenital region. It is a lymphocyte-mediated process that, in men, affects the genital skin and also the urethra, causing urethral stricture. Formerly known as balanitis xerotica obliterans (BXO), LS was first described in the late 19th century and the male form specifically by Stuhmer in 1928 (1). The term BXO had been used interchangeably with LS, however LS is currently used exclusively since the formal adoption of the term by the International Society for the Study of Vulvar Disease (2). LS is the most common cause of long pan-urethral stricture in males (3,4).
Etiology
While no exact mechanism for the formation of LS is established, there are multiple theories including infection, trauma, genetic, and autoimmune disorders. The prevalent theory currently is that LS is of autoimmune etiology (5-7), with findings of immune dysregulation being common (7). Autoantibodies as well as in an increased incidence of other autoimmune conditions have been found in patients with LS (8). In a study by Oyama et al. autoantibodies to extracellular matrix protein 1 (ECM-1) were detected in the serum of 67% of LS patients and only 7% of controls (5). Some have noted an association with HLA subtypes, specifically DQ-7 (9-11) as well as an association with other autoimmune disorders including vitiligo, alopecia arreata, and thyroid disease (12). There are no specific genes known to have influence over LS and there does not appear to be any direct genetic etiology. Multiple infectious agents have been investigated for a link to LS including Borrelia burgdorferi (13) and human papilloma virus (14-16), however there is no conclusive evidence which can show a causative relationship. Koebner described the phenomenon of psoriatic lesions at sites of cutaneous injury, suggesting trauma as a possible inciting event in the pathogenesis of LS (17,18). In spite of these myriad theories, there is still no definitive evidence pointing to any one cause as the dominant etiology for LS.
Epidemiology
LS was found to be present in 1 in 300 to 1 in 1,000 of all patients referred to a community based dermatology department (19). LS is more prevalent in women than men (6:1-10:1) and women tend to present later in life [5th to 6th decade (1)], while there is some discrepancy in the noted age of presentation in men. Some have noted a peak in the 3rd to 4th decade in men (20-22), while more recent data noted a peak prevalence in men aged 61 and older. In a review of a large population of male Department of Defense medical beneficiaries (TriCare etc), the age distribution of LS was found to be more than double in the 4th through 6th decades of life compared to first 3 decades. The highest prevalence was in ages 61 or greater (4.4 men per 100,000 visits) (23).
Clinical features
LS in males can range in severity from mild to aggressive courses. It is most commonly found in the genital region with a 5:1 ratio of genital compared with extragenital involvement (20,21). When genital LS is present, it almost always involves the foreskin/glans and, in more serious cases, the urethra may be involved. Involved skin can appear gray or white in color with cracking or fissuring of the skin in later cases. In a retrospective review of 522 patients, Depasquale et al. found disease of the prepuce and glans only in 57% of patients and the meatus in 4%. A total of 20% had urethral involvement (24). When confined to the cutaneous surfaces, LS can often be treated conservatively with topical therapies or circumcision (25), however urethral involvement often portends a poor prognosis with need for more invasive therapies. LS can be confused with erythroplasia of Querat, lichen planus, leukoplakia, and scleroderma. Local symptoms can include pruritis, dysuria, phimosis, and lower urinary tract symptoms including weak stream and frequency. In all cases, a biopsy should be obtained to confirm the diagnosis and rule out squamous cell carcinoma (SCC) as many other medical conditions may mimic LS such as various fungal infections.
In cases of urethral involvement, the stricture typically starts at the meatus and progressively moves proximally within the urethra (Figure 1). In advanced cases, spongiofibrosis and mucosal involvement can be found as proximally as the posterior urethra. The proximal extent of disease is usually well demarcated and in our practice we have yet to see any cases with skip lesions noting that all patients with LS stricture start at the meatus. A recent review of 70 patients with isolated bulbar strictures noted LS in 44% of patients on pathologic re-review suggesting LS as a possible etiology for isolated bulbar strictures (26). While the possibility for an atypical skip presentation of LS with an isolated bulbar stricture seems to exist, it has not been our experience that this is the case. Bladder involvement has yet to be described.
Because of this, when the external genitalia is involved, one needs to always think of possible involvement of the internal organs—the urethra. Therefore, evaluation of the urethral is mandatory with a combination of American Urological Association (AUA) symptom scores, retrograde urethrography, cystourethroscopy and uroflow as clinically indicated in order to determine the severity and proximal extent of disease (21,24).
Association with SCC
The rate of squamous cell carcinoma in male patients with LS has been described in case series at 2-8.6% (24,27). Penile SCC, LS and human papillomavirus (HPV) are all more common in uncircumcised men (28). Additionally, in females, LS has long been known to be associated with SCC of the vulva (16). In one series, penile cancer developed in 5.8% of a cohort of 86 men with LS. Eighty percent of the cases of penile cancer had HPV 16 detected by PCR (29), all in uncircumcised men. In another review of 20 cases of penile SCC, 10 patients (50%) were found to have histologic evidence of LS. In the same series, LS predated the diagnosis of SCC by up to 10 years, highlighting the importance of long-term follow-up in these patients (28).
Medical management
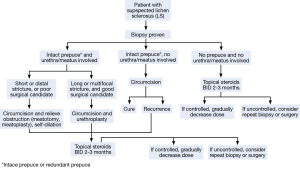
Goals of management of LS should be to alleviate symptoms, prevent and treat urethral stricture disease and prevent and detect malignant transformation (Figure 2). In our practice we have developed three goals for maintenance of male patients with genital LS:
- Goal number 1—unobstructed voiding;
- Goal number 2—painless intercourse;
- Goal number 3—adequate cosmesis (21).
We have found that when we achieve goals 1 and 2, most patients rarely wish to move forward with more invasive therapies to achieve goal number 3.
The early aggressive treatment of LS often will stop progression and may even result in regression of disease and symptoms (30). Medical management of LS traditionally begins with topical corticosteroids. Steroids will allow for the inhibition of chronic inflammation and has been shown improve symptoms and slow progression (20). In a double-blind, placebo controlled study of 40 boys with LS, 41% showed clinical improvement with steroid administration (31). Steroid preparations used include clobestasol propionate (0.05%) twice daily for 2-3 months with gradual dose lowering (32). Other options include betamethasone (0.05%), mometasone (0.1%), and hydrocortisone (2.5-10%) (33). Prescribers should be aware of potential side effects of these medications, which include cutaneous atrophy, adrenal suppression and contact sensitivity. Potent steroids should be avoided in children and hands should be washed after applying to avoid transfer to other individuals or parts of the body.
LS of the glans has also been successfully treated with the immunomodulator tacrolimus (FK506) which is a calcineurin inhibitor that blocks the production of IL-2 and T-cell activation (34). There have been encouraging studies with respect to the efficacy of tacrolimus for both primary and maintenance treatment of LS (35-37), although most agree that corticosteroids should remain first line. Other drugs which have been used for LS include topical testosterone (38,39) and systemic retinoid acitretin (40). In a randomized, double blind, placebo controlled study, oral acitretin achieved wither complete or partial response in 72.8% of patients compared to 18.8% of placebo controls (37). Other therapies that have shown some moderate success in patients with LS refractory to conventional therapy include cryotherapy, CO2 laser therapy, pulse dye laser, and ultraviolet phototherapy (1,15,20,41).
Surgical management
Whereas historically the management for LS in males relied heavily on reconstructive surgery with skin grafting and multiple serial procedures, the current methods of treating LS today rely heavily on medical and topical therapy (21). However, some cases of LS with disease progression exist in spite of aggressive medical management, and surgical intervention may be required. LS is notoriously difficult to treat and recurrences after surgical therapy are not uncommon. Surgical treatments for LS include circumcision, meatal dilation, meatotomy/meatoplasty, and urethroplasty. There is no standard therapy, and surgical management should be tailored to the specific patient. It is important to remember that surgical intervention should be reserved for cases that have failed aggressive medical therapy (Figure 2).
Circumcision
Circumcision is the surgical option of choice in those patients with LS confined to the foreskin or glans and without urethral involvement that is refractory to medical management. In one series of 287 patients who had circumcision as definitive management for LS of the foreskin and/or glans, 92% had long-term cure (24). After circumcision, mild disease to the glans may revert to normal.
Meatoplasty
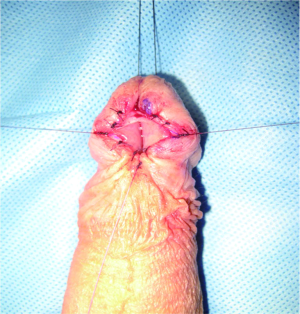
When LS is present at the meatus causing isolated meatal stenosis, ventral meatotomy or dorsal meatoplasty are the options of choice. Ventral meatotomy alone can frequently result in restenosis. Many advocate in these cases and in those with associate fossa navicularis strictures for an extended meatoplasty with creation of a hypospadic meatus. Morey et al. found extended meatoplasty to be effective in cases of refractory fossa navicularis strictures in 14 of 16 patients (87%) with complex or reoperative strictures (42). An alternative technique described by Malone includes dorsal and ventral meatotomies in conjunction with a relaxing, V-shaped incision. There were no recurrences or major complications and all patients were satisfied with cosmesis (Figure 3). Fifteen percent complained of spraying (43).
Urethroplasty
In severe cases of LS involving the anterior urethra surgical reconstruction with urethroplasty or perineal urethrostomy may be necessary. In patients with LS, urethral reconstruction should include complete excision of the involved portion of the urethra (20,21). Non-genital skin grafts should be used due to the high risk for recurrence when using genital skin. The recurrence rates when using genital skin for urethroplasty in patients with LS approach 50-100% (44,45). When re-stricturing occurs, it is usually within the first 2-3 years, however re-stricturing has been described as late as 10 years post-operatively (24). Because of this, the use of genital skin in either flap or graft form is now considered inappropriate due to high failure rates (33).
The first choice for graft material is currently buccal mucosa, which can be used for either 1- or 2-stage urethroplasty. Buccal mucosa has the advantage of a consistent, thin, and highly vascular lamina propria that makes it a very reliable graft material with excellent revascularization. 1-stage repairs for strictures from LS have been described utilizing a dorsal buccal mucosa graft when the urethral lumen is >6 Fr in diameter. For pendulous strictures, a circumcision incision is used and the penis is degloved. When the stricture extends into the bulbar urethra, a perineal incision is necessary and the penis is invaginated in the technique described by Kulkarni (46,47). The technique for dorsal onlay graft urethroplasty for LS involves the dissection of the urethra away from the corporal bodies for the entire length of the stricture. A dorsal urethrotomy is then made and the buccal graft is secured to the corpora cavernosa. The urethra is then sewn to the graft. In one series, Dubey et al. reported successes in 88% of patients over 32.5 months follow-up (48). Kulkarni et al. had a success rate of 91% in 88 patients who underwent 1-stage dorsal onlay technique for urethral stricture disease secondary to LS at a mean follow-up of 32.5 months (49). While these results are encouraging, one still needs to soberly consider the risk of recurrent disease of LS in the tissue that is not excised at the time of surgery.
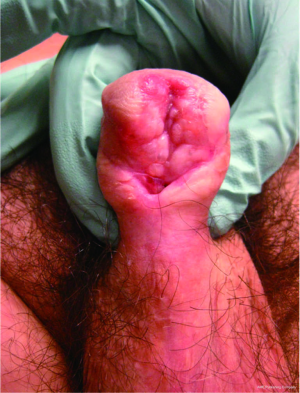
In more severe cases where the urethral plate is not salvageable, a 2-stage urethroplasty is necessary. The technique in these cases involves the complete excision of the affected urethra via a midline penile incision. In the first stage, buccal mucosa is secured to the tunica albuginea. After a period of 6-12 months, the graft is then tubularized over a catheter (50). In cases where graft take is not acceptable, or LS has recurred (Figure 4), repeating the first stage may be required. In a series by Kulkarni et al. success rate of the 2-stage buccal urethroplasty was 73% at mean follow-up of 43 months (49). In another series by Peterson et al. success was achieved in 82% of 11 patients who underwent 2-stage urethroplasty. Interestingly, in that series 8 of 19 patients (42%) who were to undergo 2-stage urethroplasty elected not to proceed to a second stage leaving them with a functioning perineal urethrostomy (51).
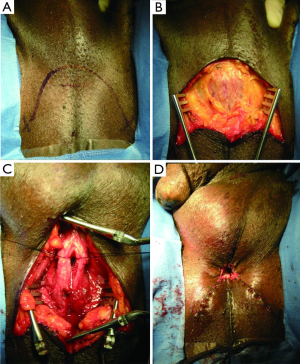
The perineal urethrostomy is increasingly considered a viable and acceptable option for the diversion of urine in this complex patient population. Additionally, recent observations indicate that many men will be happy with the perineal urethrostomy as many are already accustomed to seat voiding (51,52). The key to this procedure is the use of a wide based flap in order to produce the perineal urethrostomy (Figure 5) (53). The flap-based perineal urethrostomy can be performed either with an inverted U incision on the perineum, whereby the proximal bulbar urethra is secured to the flap, or using the “7-flap” (54). The 7-flap utilizes a laterally based perineal skin flap, which is secured to the lateral aspect of the calibrated proximal bulbar urethral stump. This differed from the Blandy procedure which utilized a posteriorly based flap to the same effect (53). Success rates for perineal urethrostomy range from 72-100% (49,51). Other tissues used in refractory cases where buccal mucosa is not available include bladder mucosa, rectal mucosa (55), and tunica vaginalis (21).
Chronic follow-up
Men treated for LS with medical management or surgical intervention need to be continuously monitored throughout their life for recurrence of symptoms or development of secondary problems such as possible SCC. We recommend seeing patients at least on a yearly basis with history, flow tests, symptom scores and careful physical examination. Continued counseling about the use of steroid medications is important as this can sometimes lead to unexpected side effects such as skin thinning, and damage other structures such as cataracts if inadvertently rubbed in the eyes.
Conclusions
LS is a chronic inflammatory disease that is often localized to the genital skin and urethra in men. It has an association with squamous cell carcinoma and early biopsy to confirm diagnosis, as well as long-term follow-up is recommended. For cases isolated to the prepuce, glans, and meatus, topical steroids are recommended. More proximal urethral disease is difficult to treat and urethral reconstruction may be necessary. When urethroplasty is performed, either 1- or 2-stage procedures are acceptable depending on the presentation and tissue replacement with buccal mucosa grafts is the standard. Genital skin should not be used in either graft or flap based repairs in patients with LS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet 1999;353:1777-83. [PubMed]
- New nomenclature for vulvar disease. Obstet Gynecol 1976;47:122-4. [PubMed]
- Kulkarni SB, Joshi PM, Venkatesan K. Management of panurethral stricture disease in India. J Urol 2012;188:824-30. [PubMed]
- Barbagli G, Mirri F, Gallucci M, et al. Histological evidence of urethral involvement in male patients with genital lichen sclerosus: a preliminary report. J Urol 2011;185:2171-6. [PubMed]
- Oyama N, Chan I, Neill SM, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet 2003;362:118-23. [PubMed]
- Sander CS, Ali I, Dean D, et al. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br J Dermatol 2004;151:627-35. [PubMed]
- Regauer S. Immune dysregulation in lichen sclerosus. Eur J Cell Biol 2005;84:273-7. [PubMed]
- Meyrick Thomas RH, Ridley CM, McGibbon DH, et al. Lichen sclerosus et atrophicus and autoimmunity--a study of 350 women. Br J Dermatol 1988;118:41-6. [PubMed]
- Marren P, Yell J, Charnock FM, et al. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol 1995;132:197-203. [PubMed]
- Azurdia RM, Luzzi GA, Byren I, et al. Lichen sclerosus in adult men: a study of HLA associations and susceptibility to autoimmune disease. Br J Dermatol 1999;140:79-83. [PubMed]
- Aslanian FM, Marques MT, Matos HJ, et al. HLA markers in familial Lichen sclerosus. J Dtsch Dermatol Ges 2006;4:842-7. [PubMed]
- Bjekić M, Šipetić S, Marinković J. Risk factors for genital lichen sclerosus in men. Br J Dermatol 2011;164:325-9. [PubMed]
- Edmonds E, Mavin S, Francis N, et al. Borrelia burgdorferi is not associated with genital lichen sclerosus in men. Br J Dermatol 2009;160:459-60. [PubMed]
- Drut RM, Gómez MA, Drut R, et al. Human papillomavirus is present in some cases of childhood penile lichen sclerosus: an in situ hybridization and SP-PCR study. Pediatr Dermatol 1998;15:85-90. [PubMed]
- Beattie PE, Dawe RS, Ferguson J, et al. UVA1 phototherapy for genital lichen sclerosus. Clin Exp Dermatol 2006;31:343-7. [PubMed]
- Neill SM, Lessana-Leibowitch M, Pelisse M, et al. Lichen sclerosus, invasive squamous cell carcinoma, and human papillomavirus. Am J Obstet Gynecol 1990;162:1633-4. [PubMed]
- Miller RA. The Koebner phenomenon. Int J Dermatol 1982;21:192-7. [PubMed]
- Pock L. Koebner phenomenon in lichen sclerosus et atrophicus. Dermatologica 1990;181:76-7. [PubMed]
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc 1971;57:9-30. [PubMed]
- Das S, Tunuguntla HS. Balanitis xerotica obliterans--a review. World J Urol 2000;18:382-7. [PubMed]
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol 2007;178:2268-76. [PubMed]
- Kizer WS, Prarie T, Morey AF. Balanitis xerotica obliterans: epidemiologic distribution in an equal access health care system. South Med J 2003;96:9-11. [PubMed]
- Nelson DM, Peterson AC. Lichen sclerosus: epidemiological distribution in an equal access health care system. J Urol 2011;185:522-5. [PubMed]
- Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int 2000;86:459-65. [PubMed]
- Dahlman-Ghozlan K, Hedblad MA, von Krogh G. Penile lichen sclerosus et atrophicus treated with clobetasol dipropionate 0.05% cream: a retrospective clinical and histopathological study. J Am Acad Dermatol 1999;40:451-7. [PubMed]
- Liu JS, Walker K, Stein D, et al. Lichen sclerosus and isolated bulbar urethral stricture disease. J Urol 2014;192:775-9. [PubMed]
- Barbagli G, Palminteri E, Mirri F, et al. Penile carcinoma in patients with genital lichen sclerosus: a multicenter survey. J Urol 2006;175:1359-63. [PubMed]
- Powell J, Robson A, Cranston D, et al. High incidence of lichen sclerosus in patients with squamous cell carcinoma of the penis. Br J Dermatol 2001;145:85-9. [PubMed]
- Barbagli G, De Angelis M, Palminteri E, et al. Failed hypospadias repair presenting in adults. Eur Urol 2006;49:887-94; discussion 895. [PubMed]
- Tausch TJ, Peterson AC. Early aggressive treatment of lichen sclerosus may prevent disease progression. J Urol 2012;187:2101-5. [PubMed]
- Kiss A, Csontai A, Pirót L, et al. The response of balanitis xerotica obliterans to local steroid application compared with placebo in children. J Urol 2001;165:219-20. [PubMed]
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol 2005;48:808-17. [PubMed]
- Stewart L, McCammon K, Metro M, et al. SIU/ICUD Consultation on Urethral Strictures: Anterior urethra-lichen sclerosus. Urology 2014;83:S27-30. [PubMed]
- Pandher BS, Rustin MH, Kaisary AV. Treatment of balanitis xerotica obliterans with topical tacrolimus. J Urol 2003;170:923. [PubMed]
- Kyriakou A, Patsialas C, Patsatsi A, et al. Treatment of male genital lichen sclerosus with clobetasol propionate and maintenance with either methylprednisolone aceponate or tacrolimus: a retrospective study. J Dermatolog Treat 2013;24:431-4. [PubMed]
- Ebert AK, Rösch WH, Vogt T. Safety and tolerability of adjuvant topical tacrolimus treatment in boys with lichen sclerosus: a prospective phase 2 study. Eur Urol 2008;54:932-7. [PubMed]
- Hengge UR, Krause W, Hofmann H, et al. Multicentre, phase II trial on the safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. Br J Dermatol 2006;155:1021-8. [PubMed]
- Ayhan A, Guven S, Guvendag Guven ES, et al. Topical testosterone versus clobetasol for vulvar lichen sclerosus. Int J Gynaecol Obstet 2007;96:117-21. [PubMed]
- Pasieczny TA. The treatment of balanitis xerotica obliterans with testosterone propionate ointment. Acta Derm Venereol 1977;57:275-7. [PubMed]
- Ioannides D, Lazaridou E, Apalla Z, et al. Acitretin for severe lichen sclerosus of male genitalia: a randomized, placebo controlled study. J Urol 2010;183:1395-9. [PubMed]
- Kastner U, Altmeyer P. Cryosurgery--the last resort or a surgical alternative in the treatment of lichen sclerosus et atrophicus of the vulva (LSAV)? J Dtsch Dermatol Ges 2003;1:206-11. [PubMed]
- Morey AF, Lin HC, DeRosa CA, et al. Fossa navicularis reconstruction: impact of stricture length on outcomes and assessment of extended meatotomy (first stage Johanson) maneuver. J Urol 2007;177:184-7; discussion 187. [PubMed]
- Malone P. A new technique for meatal stenosis in patients with lichen sclerosus. J Urol 2004;172:949-52. [PubMed]
- Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol 1998;81:735-7. [PubMed]
- Virasoro R, Eltahawy EA, Jordan GH. Long-term follow-up for reconstruction of strictures of the fossa navicularis with a single technique. BJU Int 2007;100:1143-5. [PubMed]
- Kulkarni S, Barbagli G, Sansalone S, et al. One-sided anterior urethroplasty: a new dorsal onlay graft technique. BJU Int 2009;104:1150-5. [PubMed]
- Kulkarni SB, Kulkarni JS, Kirpekar DV. A new technique of urethroplasty for balanitis xerotica obliterans. J Urol 2000;163:352.
- Dubey D, Sehgal A, Srivastava A, et al. Buccal mucosal urethroplasty for balanitis xerotica obliterans related urethral strictures: the outcome of 1 and 2-stage techniques. J Urol 2005;173:463-6. [PubMed]
- Kulkarni S, Barbagli G, Kirpekar D, et al. Lichen sclerosus of the male genitalia and urethra: surgical options and results in a multicenter international experience with 215 patients. Eur Urol 2009;55:945-54. [PubMed]
- Barbagli G. When and how to use buccal mucosa grafts in penile and bulbar urethroplasty. Minerva Urol Nefrol 2004;56:189-203. [PubMed]
- Peterson AC, Palminteri E, Lazzeri M, et al. Heroic measures may not always be justified in extensive urethral stricture due to lichen sclerosus (balanitis xerotica obliterans). Urology 2004;64:565-8. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. Clinical outcome and quality of life assessment in patients treated with perineal urethrostomy for anterior urethral stricture disease. J Urol 2009;182:548-57. [PubMed]
- Myers JB, McAninch JW. Perineal urethrostomy. BJU Int 2011;107:856-65. [PubMed]
- French D, Hudak SJ, Morey AF. The "7-flap" perineal urethrostomy. Urology 2011;77:1487-9. [PubMed]
- Vanni A, Marcello PW, Zinman LN. V2-02 trans-anal colonic mucosa graft onlay for salvage urethroplasty. J Urol 2014;191:e357.