Con: bulbomembranous anastomotic urethroplasty for pelvic fracture urethral injuries
IntroductionOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Acute management of pelvic fracture urethral injury (PFUI) remains a controversial and difficult decision for urologists. As the literature is comprised of predominantly case series, meaningful comparisons of primary endoscopic realignment (PER) vs. suprapubic tube (SPT)/bulbomembranous anastomotic urethroplasty (BMAU) are challenging (1,2). Some surgeons prefer to leave the SPT for several months, followed by an elective BMAU. Others attempt to re-establish urethral continuity early with realignment. A potential benefit of PER is avoidance of the need for urethroplasty altogether, or a decrease in the technical difficulty of additional procedures, though this too remains debatable.
SPT placement alone almost inevitably results in urethral stenosis in PFUI patients (3), but it is nearly always amenable to BMAU after a period of time allowing for tissue healing. In the large proportion of patients in whom PER fails, multiple interval interventions often subject them to painful office dilations, lost time from work, and significant delay in return to unobstructed voiding (4). At our tertiary care institution, patients are routinely referred from outside facilities having undergone either form of treatment, and those who were primarily realigned endured significantly more procedures and longer time-intervals before re-establishment of their urethral continuity.
PER-related complicationsOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Proponents of PER advocate the potential for earlier return to voiding with obviation of the need for future urethral reconstruction. Additionally, the strictures that do develop may be shorter and the urethra better aligned for the subsequent urethroplasty (5,6). However, our experience has found that neither result is achieved, with no difference in average stricture length or mean operative time in PER vs. SPT/BMAU patients (4).
Although literature over the preceding half century has been variable and inconclusive, PER does appear to reduce the risk of urethral stricture development by about 30% (7). In a recent meta-analysis of published reports over the last three decades, the authors concluded that PER reduces stricture rates by 37.2%, with a number needed to treat of 2.76 (8). Recent single-institution studies corroborate these results with success rates ranging between 14% and 45% (9-11). PER patients do tend to maintain some degree of urethral patency, however we have noticed that they tend to undergo numerous endoscopic or office procedures over lengthy periods of time—often continued for years or even decades (4).
Repeated endoscopic procedures in patients with anterior (bulbar) stricture disease result in similar delays. Hudak et al. reported a delay of nearly 16 years in patients who underwent two or more endoscopic repairs prior to urethroplasty, compared to only 2 years for those receiving 0-1 previous treatments; increasing complexity and difficulty of repair was also noted following repeated instrumentation (12). Park and McAninch also noted that straddle injuries treated by PER tended to require more (not less) complicated repairs than those treated by SPT/BMAU (13).
Urethral strictures after PEROther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
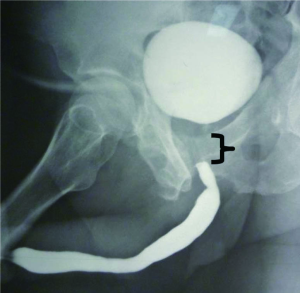
The acute nature of a PFUI can be turned into a chronic unstable state by PER, with the resultant tenuous urethral patency requiring daily self-dilation or multiple office procedures. Men strongly prefer not to be subjected to such interventions, often fearing the threat of recurrent urinary retention and accompanying interventions (14). “Minimally- invasive” procedures are generally futile, and urethrotomy has only a transient efficacy, with low success rates published at less than 10% (15). As the urethra is a delicate anatomic structure, increasing the frequency of dilation-associated assault inflicts unnecessary tissue injury, resulting in pain, false passages (Figure 1), bleeding, and lost time from work.

Furthermore, PER followed by repeated instrumentation seemed to complicate performance of posterior urethroplasty, an already challenging procedure. Repeated dilations can propagate scar formation, increasing periurethral fibrosis (12). At our institution, we also noted a wide range of adverse events in PER patients: synchronous stricture formation, false passages, initial urethroplasty failure, and/or infectious complications. Admittedly, these complications were likely not related to PER itself, but rather to the subsequent aggressive endoscopic manipulations required following impending urinary retention after being lost to follow up (4).
We acknowledge that PER may be successful in select cases, resulting in durable urethral patency. However, close follow-up and/or prompt referral to a reconstructive subspecialist in the event of stricture formation is imperative. Despite most authorities recommending routine evaluations following PER (11), it is clear that many men are being lost to follow-up and/or dilated indefinitely without referral to a subspecialist. Fortunately, in experienced hands, BMAU is effective as a salvage strategy regardless of initial and subsequent management.
SPT and BMAUOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Extreme forces are necessary to cause a pelvic fracture, and patients often sustain concomitant, life-threatening injuries that warrant emergent intervention. Emergent management of the bladder can be safely accomplished with placement of an SPT, and though concern for infectious complications in the setting of orthopedic hardware sometimes precludes the procedure, to our knowledge there is no scientific literature to support this claim. After placement of an SPT (which can be done at the time of laparotomy or percutaneously), patients can recover from the initial insult and be referred to a specialist for further management. Although a urethral stricture is almost inevitable, urethroplasty can be performed in a controlled setting after allowing for tissue healing (16-20).
Complete stricture excision with primary tension-free anastomosis (EPA) is the gold standard approach for resolution of obstructive urethral lesions. Contemporary success rates of anastomotic urethroplasty for both PFUIs and for bulbar strictures are reported in the range of 93% (3,21). In a canine experiment from 1970, McRoberts and Ragde severed the urethras of 22 dogs and repaired the defects by precise mucosal apposition with sutured anastomoses in half and compared subsequent stricture rates to those realigned with catheters alone (22). Nearly all (10/11, 91%) urethras stented with catheters only developed strictures, while none of the repaired urethras stenosed. Histological analysis confirmed that catheter stenting alone results in retraction of the severed urethral ends into periurethral tissue, caused by contraction of the intimately associated muscle layer. The intervening gap was not replaced by re-epithelialization, but rather by fibrotic scar, as is the case with PFUIs.
At our institution, delayed urethroplasty after SPT is the preferred management algorithm for PFUI. Posterior urethroplasty has been successful for resolution of stricture in 100% of patients initially managed by SPT and in 76% of patients who presented with strictures following attempts at PER (4). Though stricture length was similar between these two groups (2.6 vs. 2.8 cm, respectively), those patients undergoing PER had a significantly longer time-course to durable resolution (mean of 7 vs. 25 months, respectively). PER patients also experienced a median of four endoscopic interventions prior to definitively urethroplasty. Endoscopic realignment may be an appropriate management strategy in the acute setting; however, urologists should carefully consider the better and timelier outcomes of SPT placement and referral for definitive posterior urethroplasty.
BMAU techniqueOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Following initial management with SPT, anastomotic urethroplasty is typically performed after 2-3 months. This period allows for tissue healing and reabsorption of the resultant hematoma, which is replaced by fibrous tissue (23-25). The principles of anastomotic urethroplasty must be followed: epithelial apposition, establishment and/or preservation of blood supply to the anastomosis, resection of scar providing healthy tissue to hold sutures, and preservation of bladder neck competence. Usually the end result of a PFUI is a relatively short bulbomembranous gap, amenable to a primary anastomosis (Figure 2).
In rare cases of high prostatic dislocation, longer defects of >2-3 cm may result. For these cases, various maneuvers have traditionally been employed. Although a step-wise approach described by Webster has been established (26), we have found that corporal splitting is rarely necessary, and the vasculature can be spared providing better support of the anastomosis. Aggressive distal urethral mobilization nearly always yields enough length to achieve a tension-free anastomosis, and in severe cases an inferior pubectomy can shorten the distance in order to complete the repair (19).
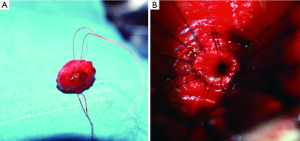
The BMAU technique involves a midline perineal incision. The urethra is divided as proximally as possible, at the distal-most aspect of the resultant scar tissue from the hematoma. The urethra is then mobilized distally, allowing sufficient length to bridge the gap. The fibrotic scar is completely excised (Figure 3A), revealing healthy urothelium for the proximal anastomosis. Anastomotic sutures of 5-0 polydioxanone (PDS) sutures on RB-1 needles in a 12-suture, “clock-face” configurations are used to reapproximate the mucosa (Figure 3B). Double-armed sutures are beneficial in ensuring mucosal edges are incorporated, while also allowing for the knots to be tied on the extra-luminal side of the urethra. A 16 French Foley catheter is then left for 3 weeks to allow for tissue healing.
LimitationsOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
When reviewing the literature, it is difficult to make definitive statements regarding the superiority of either PER or SPT/BMAU for acute management of PFUI. Published reports include different methods of achieving realignment, variable definitions of success, and there is as of yet no prospective, controlled trial comparing the two methods. Injury severity may affect outcomes as well, and this is also not well-reported in the literature.
PER may avoid open urethral reconstruction in a minority of patients, but it is impossible to predict who will fail and require operative repair. Our institutional experience has demonstrated the potential pitfalls that result from PER patients being lost to follow-up and/or subjected to multiple interval procedures, and for this reason we prefer to place an SPT in the acute setting and perform an elective BMAU in a controlled setting allowing ample time for tissue healing.
Additionally, with emphasis on decreasing economic burden to the healthcare system, physicians must provide the best possible care while reducing unnecessary costs. To our knowledge, no reports include a cost-analysis, but one can assume that the additional costs associated with the added interval follow-up visits, office procedures, and operative interventions required for PER patients would likely significantly outweigh the cost of patients undergoing SPT/BMAU.
ConclusionsOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Our experience has demonstrated the potential for PFUI patients to endure complex and prolonged treatment courses following PER. The multiple interval procedures necessary to maintain urethral patency can subject patients to unnecessary pain, time lost from work, and cost, while delaying their recovery. SPT placement and referral to specialized centers may significantly reduce the time course to return of unobstructed voiding and decrease the burden on our healthcare system.
AcknowledgementsOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
None.
FootnoteOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Introduction
- PER-related complications
- Urethral strictures after PER
- SPT and BMAU
- BMAU technique
- Limitations
- Conclusions
- Acknowledgements
- Footnote
- References
- Koraitim MM. Pelvic fracture urethral injuries: the unresolved controversy. J Urol 1999;161:1433-41. [PubMed]
- Webster GD, Mathes GL, Selli C. Prostatomembranous urethral injuries: a review of the literature and a rational approach to their management. J Urol 1983;130:898-902. [PubMed]
- Gomez RG. Stricture excision and primary anastomosis for anterior urethral strictures. In: Brandes SB, Morey AF. eds. Advanced Male Urethral and Genital Reconstructive Surgery, 2nd ed. New York: Humana Press, 2013:161-76.
- Tausch TJ, Morey AF, Scott JF, et al. Unintended negative consequences of primary endoscopic realignment for men with pelvic fracture urethral injuries. J Urol 2014;192:1720-4. [PubMed]
- Ku Ja, Kim ME, Jeon YS, et al. Management of bulbous urethral disruption by blunt external trauma: the sooner, the better? Urology 2002;60:579-83. [PubMed]
- Mouraviev VB, Coburn M, Santucci RA. The treatment of posterior urethral disruption associated with pelvic fractures: comparative experience of early realignment versus delayed urethroplasty. J Urol 2005;173:873-6. [PubMed]
- Gómez RG, Mundy T, Dubey D, et al. SIU/ICUD Consultation on Urethral Strictures: Pelvic fracture urethral injuries. Urology 2014;83:S48-58. [PubMed]
- Barrett K, Braga LH, Farrokhyar F, et al. Primary realignment vs suprapubic cystostomy for the management of pelvic fracture-associated urethral injuries: a systematic review and meta-analysis. Urology 2014;83:924-9. [PubMed]
- Hadjizacharia P, Inaba K, Teixeira PG, et al. Evaluation of immediate endoscopic realignment as a treatment modality for traumatic urethral injuries. J Trauma 2008;64:1443-9; discussion 1449-50. [PubMed]
- Sofer M, Mabjeesh NJ, Ben-Chaim J, et al. Long-term results of early endoscopic realignment of complete posterior urethral disruption. J Endourol 2010;24:1117-21. [PubMed]
- Leddy LS, Vanni AJ, Wessells H, et al. Outcomes of endoscopic realignment of pelvic fracture associated urethral injuries at a level 1 trauma center. J Urol 2012;188:174-8. [PubMed]
- Hudak SJ, Atkinson TH, Morey AF. Repeat transurethral manipulation of bulbar urethral strictures is associated with increased stricture complexity and prolonged disease duration. J Urol 2012;187:1691-5. [PubMed]
- Park S, McAninch JW. Straddle injuries to the bulbar urethra: management and outcomes in 78 patients. J Urol 2004;171:722-5. [PubMed]
- Lubahn JD, Zhao LC, Scott JF, et al. Poor quality of life in patients with urethral stricture treated with intermittent self-dilation. J Urol 2014;191:143-7. [PubMed]
- Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol 2010;183:1859-62. [PubMed]
- Salehipour M, Khezri A, Askari R, et al. Primary realignment of posterior urethral rupture. Urol J 2005;2:211-5. [PubMed]
- Morey AF, McAninch JW. Reconstruction of posterior urethral disruption injuries: outcome analysis in 82 patients. J Urol 1997;157:506-10. [PubMed]
- Koraitim MM. Assessment and management of an open bladder neck at posterior urethroplasty. Urology 2010;76:476-9. [PubMed]
- Kizer WS, Armenakas NA, Brandes SB, et al. Simplified reconstruction of posterior urethral disruption defects: limited role of supracrural rerouting. J Urol 2007;177:1378-81; discussion 1381-2. [PubMed]
- Lumen N, Hoebeke P, Troyer BD, et al. Perineal anastomotic urethroplasty for posttraumatic urethral stricture with or without previous urethral manipulations: a review of 61 cases with long-term followup. J Urol 2009;181:1196-200. [PubMed]
- Cooperberg MR, McAninch JW, Alsikafi NF, et al. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol 2007;178:2006-10; discussion 2010.
- McRoberts JW, Ragde H. The severed canine posterior urethra: a study of two distinct methods of repair. J Urol 1970;104:724-9. [PubMed]
- Lynch TH, Martínez-Piñeiro L, Plas E, et al. EAU guidelines on urological trauma. Eur Urol 2005;47:1-15. [PubMed]
- Martínez-Piñeiro L, Djakovic N, Plas E, et al. EAU Guidelines on Urethral Trauma. Eur Urol 2010;57:791-803. [PubMed]
- Chapple C, Barbagli G, Jordan G, et al. Consensus statement on urethral trauma. BJU Int 2004;93:1195-202. [PubMed]
- Webster GD, Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach: experience with 74 cases. J Urol 1991;145:744-8. [PubMed]


