New frontiers in urethral reconstruction: injectables and alternative grafts
Introduction
The management of urethral strictures is varied and includes both endoscopic and open urethral reconstruction, with the goal of improving urinary flow. Urethral strictures resulting from trauma, iatrogenic injury, prior hypospadias surgery, lichen sclerosis and previous urethral reconstruction often result in long segments of urethra that require extensive reconstruction. The optimal management of a urethral stricture is dependent on a multitude of factors including the location, length, and etiology of stricture, as well as the surgeon’s experience and reconstructive preference. Urethral strictures are often managed in a progressive manner in which shorter strictures are managed endoscopically or with excision and anastomosis, while augmentation with oral mucosa grafts or fasciocutaneous flaps are necessary for longer defects.
While the buccal mucosa graft is the workhorse of urethral reconstruction, a need exists for alternative grafts for augmentation urethroplasty in patients in whom obtaining adequate graft material is difficult; namely long segment, panurethral strictures or recurrent strictures in patients who have had prior buccal mucosa graft retrieval. Buccal mucosa is now used so frequently, and with such high success, that many of the grafts we used in the past (genital and extragenital skin), are a great source to re-examine in the future. Additionally, tissue engineering and regenerative medicine offers the potential to significantly reduce the invasiveness and morbidity of urethral reconstruction by having off the shelf material available, with the goal of improving patient outcomes.
Injectables
The goal of treating any urethral stricture is to provide a durable, patent urethra with minimal morbidity. While open urethroplasty is the gold standard treatment for anterior urethral strictures, endoscopic management does have a role in highly select patients. Since the outcomes of endoscopic treatment are vastly inferior to urethroplasty, improving our current endoscopic treatments is important, and if successful, has the potential to greatly reduce patient morbidity. There have been a number of different techniques and anti-fibrotic agents used in the hope of blunting wound contraction and scar formation.
The optimal therapeutic intervention is to augment a traditional urethrotomy with an antiproliferative, anti-scar forming agent (Figures 1-3). Steroids (triamcinolone), mitomycin C (MMC), and hyaluronidase are all antifibrotic agents that have the potential to reduce stricture recurrence. Steroids have well known anti-fibrotic and anti-collagen properties. MMC has been shown in both in vitro and animal studies to inhibit fibroblast proliferation, collagen deposition, and scar formation (1-3). Additionally, MMC has been used in numerous medical specialties for its anti-scar properties. It is used for glaucoma and pterygium excision, nasolacrimal duct obstruction, laryngeal and tracheal stenosis, and vaginal and anal stenosis (4-12). Hyaluronidase is an antifibrotic agent used in hypertrophic scar, keloid, and pulmonary fibrosis. This agent suppresses wound healing inflammatory mediators, decreases fibroblast proliferation, and decreases collagen and glycosaminoglycan synthesis (13).
The current urologic literature demonstrates few high quality studies investigating the use of antifibrotic injectables for the treatment of anterior urethral strictures. Triamcinolone is the agent that has been studied the most extensively, with original reports going back to the 1960s and 1970s (14,15). Steroid injection following urethrotomy has been advocated in a number of small studies to be safe and efficacious (16,17). There has been two small randomized, controlled trials looking at bulbar strictures <1.5 cm treated with urethrotomy with and without triamcinolone (16,18). With a mean follow-up of 13.7 months (range, 1-25 months), Mazdak et al. found the triamcinolone group had a stricture recurrence rate of 21.7% as compared to 50% in the control arm. In contrast, the study by Tavakkoli et al. did not find a statistically significant difference in the rate of stricture recurrence (16).
MMC is another antifibrotic agent that has recently been studied. A small randomized, controlled trial of 40 patients with bulbar urethral strictures <1.5 cm was the first study looking at the effects of MMC on urethrotomy. Stricture recurrence was 10% in patients with urethrotomy and MMC versus 50% with urethrotomy alone. However, these results are to be taken with caution, as the follow-up was only 6-24 months and the end point for success poorly defined (19).
Hyaluronic acid and caroboxymethylcellulose have been proposed to minimize stricture recurrence in one small randomized controlled trial, but no conclusions about the efficacy of this drug can be made as the trial only had a 6 month follow-up (20).
Lastly, the combination of all three of these drugs has recently been proposed as a means of improving urethrotomy outcomes. In a study of 103 patients with strictures of both the bulbar and penile urethra, the authors performed an urethrotomy and injected a mixture of 40 mg triamcinolone, 2 mg MMC, and 3,000 units of hyaluronic acid into the urethrotomy site (21). They report a stricture recurrence rate of 19.4% at a median follow-up of 14 months (range, 3-18 months), with no control group (21).
While all of these studies are an important step in progressing the science and knowledge of endoscopic stricture management, longer follow-up and more rigid outcomes measures will be critical to properly evaluate the role these adjuncts play with regards to traditional treatment. Well-controlled clinical trials with a minimum 2-year follow-up are needed to determine the optimal antifibrotic agent and technical approach for these novel adjuncts to endoscopic management.
Alternative grafts
While the success rate of both grafts and penile skin flaps has been established in the literature to be of similar efficacy, there is also significantly higher morbidity associated with the use of such flaps (22,23). The increased complexity in harvesting penile skin flaps requires technical expertise and has resulted in the use of full thickness grafts as the augmentation tissue of choice among reconstructive surgeons. There are a number of alternative grafts that can be effectively implemented into the reconstructive urologist’s armamentarium for use in particularly complex cases, when there is insufficient buccal mucosa available for augmentation urethroplasty. Lingual grafts, penile and extragenital skin, bladder epithelium, small intestinal submucosa, colonic mucosa, and tissue engineered grafts are all existing potential sources of graft material for patients needing additional tissue for augmentation urethroplasty.
Lingual grafts
Oral mucosa grafts are the standard grafting material for augmentation urethroplasty as they are accustomed to being wet, and may be resistant to lichen sclerosis (24,25). While there is little data regarding lingual mucosa grafts, the characteristics that make buccal mucosa appealing apply to lingual mucosa grafts as well. These full thickness grafts have a thick epithelium, thin lamina propria, and rich, panlaminar vascular plexus, which allows excellent graft take with minimal contracture. The macroscopic architecture of these grafts is identical to buccal mucosa, making this the graft of choice when additional material is needed (26).
There are notable advantages to harvesting lingual grafts. They are readily available for harvest, have a concealed donor site scar, and can yield two grafts ranging between 7 and 16 cm in length. During graft harvesting, care is taken to dissect between the mucosa and submucosal fat. Both Wharton’s duct and the lingual nerve are identified, and care is taken to avoid harvesting mucosa from the floor of the mouth to maintain tongue mobility (27). Lingual grafts can be harvested from any of three locations on the tongue, the lateral surface, ventral surface, or both the ventral and lateral surface (26-28). Harvesting from the ventral location allows the potential for two grafts to be harvested if necessary. After graft harvest, the donor site is closed with absorbable suture and the graft prepared by removing the underlying fibrovascular tissue.
The urethroplasty techniques used with lingual mucosa grafts are similar to buccal grafts, with published reports of one-stage dorsal onlay, dorsal inlay, ventral onlay grafts, as well as a two-stage approach. The short-term outcomes of lingual mucosa grafts appear to be equivalent to buccal mucosa, although there are only a few published reports in the literature (26,27,29,30). Sharma et al. performed a prospective comparative analysis of lingual mucosa grafts compared to buccal mucosa grafts in 30 patients who underwent a dorsal onlay urethroplasty (31). The stricture free outcomes were similar at a mean follow-up of 14.5 months. Additionally, there have been a few additional small studies demonstrating success rates of 79-90% with lingual grafts. However the stricture etiology and surgical techniques were heterogeneous in these small studies and follow-up was less than 2 years (26-30). Lingual mucosa grafts are an excellent graft material for patients with long strictures due to lichen sclerosis, when there may be an inadequate amount of buccal mucosa available. Das et al. was the first to describe short-term results in 18 patients with lichen sclerosis, and demonstrated an 83.3% success rate in patients with a mean stricture length of 10.2 cm (29). Similarly, Xu et al. described their experience with one-stage lingual mucosa grafts in 22 patients with lichen sclerosis strictures and demonstrated an 88.9% success rate (mean stricture length 12.5 cm) with a mean follow-up of 38.7 months (32).
One of the important factors needing validation is the donor site morbidity associated with lingual mucosa harvest. It appears from the small number of studies published, that complications are rare. Initial reports by Simonato et al. reported that patients only complained of slight donor sight pain for 1-2 days, while Das et al. found minimal complications with no functional or esthetic deficiency (29,33). Kumar et al. found that all patients were pain free by 6 days, while in patients with bilateral tongue harvest, they temporarily had a higher rate of slurred speech (34). Sharma et al. published donor site morbidity with long-term changes in speech in patients who underwent bilateral lingual harvest for strictures >7 cm (31).
Lingual grafts represent an excellent choice as an alternative graft to buccal mucosa in patients requiring long-segment substitution urethroplasty, in patients who have previously had buccal mucosa harvest, or in patients in whom cheek harvest would otherwise be contraindicated. While lingual grafts share all the important characteristics of buccal mucosa, the long-term outcomes of lingual grafts have not been established, as the longest follow-up published to date is 38.7 months. Additionally, while harvesting lingual grafts appears safe, the optimal graft size and location on the tongue has not been established to minimize potential long-term morbidity.
Genital and extragenital skin
While skin grafts are certainly not a “New frontier” in urethral reconstruction, there is an important use in modern urethral reconstruction for these grafts in patients without lichen sclerosis. Substitution urethroplasty with a penile skin graft was first described in 1953 by Presman and Greenfield, and was ultimately promoted by Devine and Horton using preputial skin for hypospadias repairs (35-37). Although the use of penile and extragenital skin grafts has been declining since early 1990s, the use of full thickness skin grafts remains an integral part of the reconstructive urologist’s armamentarium. Certain conditions exist which ideally suit the harvest of skin (oral leukoplakia, heavy tobacco use, chewing tobacco, betel nut or pan masala, previous oral radiotherapy, previous oral mucosa grafting, insufficient oral mucosa) for substitution urethroplasty. Penile skin grafts are hairless, elastic, and easy to harvest with minimal donor site morbidity. A recent meta-analysis be Lumen et al. comparing urethral reconstruction with either a penile skin or buccal mucosa demonstrated a success rate of 81.8% vs. 85.9% respectively, P=0.01 (38). The long-term durability of penile skin grafts could not be assessed in this analysis, as the follow-up was only 64 months. However, a recent publication by Barbagli et al. demonstrated the long-term outcomes of 359 patients who had either an oral mucosa or penile skin graft urethroplasty. With a minimum follow-up of 6 years, patients with penile skin grafts had a success rate of 59.7% as compared to 77.7% of patients with an oral mucosa graft (39).
Postauricular skin is a full thickness graft that can be used for the treatment of anterior urethral strictures when oral mucosa and genital skin are not available. The skin is harvested from the lower half of the mastoid, and should not cross beyond the lower end of the tragus. Manoj et al. has demonstrated the ability to harvest 7-8 cm of full-thickness graft per side, allowing 14-16 cm of graft material if necessary. In 35 patients with a follow-up of 21 months, they demonstrated an 89% success rate, and reported no donor site complications (40). If harvesting this graft, consideration of the donor site scar should be taken into consideration.
Abdominal skin is another full-thickness skin graft alternative to postauricular skin. This graft should be taken from a hairless area in either the flank or right or left lower quadrant, near the level of the anterior iliac spine. The donor site is closed and the graft prepared by removing all the areolar tissue. The indications for use are similar to postauricular grafts, namely patients without lichen sclerosis in whom oral mucosa and genital skin are not available. A potential use of full thickness skin was described Chen et al. who used a combination of full-thickness skin grafts dorsally in combination with ventral buccal mucosa graft for long segment strictures and reported a 100% success rate in patients with strictures >6 cm with the double graft technique (41). Further evaluation and longer follow-up will be needed to confirm the preliminary findings of this study.
Another extragenital skin graft option for the treatment of particularly complex, long strictures with severe spongiofibrosis is the use of a meshed split-thickness skin graft. Popularized by Schreiter, these grafts are used in a two-stage approach (42). In expert hands, these grafts perform exceedingly well in the most complex of cases, with a 79% success rate at 6.5 years follow-up (43). The overall complication rate with a two stage meshed graft urethroplasty is low, with erectile dysfunction and penile curvature rates reported to occur in 4% and 9% of patients respectively (44). While these grafts were used before the modern era of buccal mucosa, they currently represent a tiny fraction of urethroplasties performed. However, the mesh graft technique is a useful alternative for the most complex urethral stricture.
Bladder mucosa
Bladder mucosa is a free graft that is available and has been used primarily in the setting of hypospadias surgery. This graft has fallen out of favor both because of the invasive nature of harvest required to obtain the graft, as well as the high rate of stricture recurrence. In particular, meatal problems occur in up to 68% of patients when the graft was exposed to air, and up to 66% of patients required multiple operations to achieve a good result (45).
Colonic mucosa
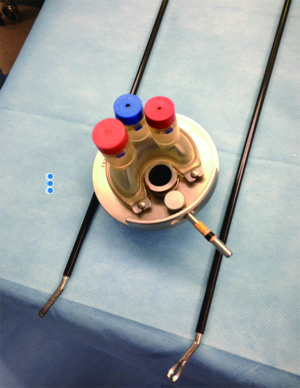
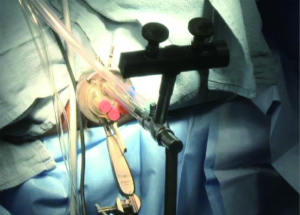
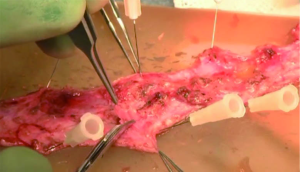
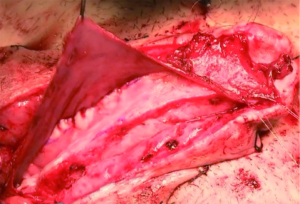
While Mundy et al. has described the successful use of intestinal flaps as a salvage procedure for bulbomembranous urethral strictures, colonic mucosa has also been used as a circumferential graft in patients with long segment urethral strictures (46,47). Xu et al. reported an 85.7% success rate in 36 patients with a follow-up of 53.6 months (mean stricture length 15.1 cm). However, these patients all underwent a sigmoid resection in order to obtain the graft, raising questions about the practicality of such a technique (47). We have found that retrieving colonic mucosa for salvage urethroplasty is possible without a bowel resection with a transanal endoscopic micro-surgical technique (TEMS) (Figures 4-8). The TEMS technique was developed by colorectal surgeons for the minimally invasive treatment of early stage rectal tumors and benign polyps high in the rectum (up to 20 cm). We have successfully performed this technique safely in four patients for salvage urethroplasty, with no perioperative complications (Vanni and Zinman unpublished data). At a mean follow-up 13 months (range, 5-19 months), three of the four patients have had a successful outcome, while one patient has required a urethral dilation for recurrent stricture. All four patients have normal bowel function. However, larger patient numbers and long-term follow-up will be important in determining what, if any, role colonic mucosa has in salvage urethroplasty.
Acellular matrix/tissue engineering
There is great promise in the field of tissue engineering for the development of an off the shelf graft suitable for urethral reconstruction. While oral mucosa grafts are readily available and adequate for the vast majority of strictures, alternative grafts are needed in the most complex cases. Small intestinal submucosa is a prefabricated, acellular, collagen matrix manufactured from porcine intestinal submucosa. Initial, short-term results in two pilot studies demonstrated success rates between 90-100% (48,49). However, at longer follow-up in 25 patients, success was 86% for strictures <4 cm, while in strictures >4 cm no patient had a successful reconstruction (50). Much research has been performed trying to identify the best scaffold and cell for replacing strictures of the urethra. Autologous tissue engineered buccal mucosa is a promising substitute, and in a pilot study of five patients, all had an initial good result, and at 3 years follow-up, three of the patients had patent urethras (51). Additionally, synthetic scaffolds have been studied as an off the shelf alternative to human or animal collagen scaffolds. In a study by Raya-Rivera et al., five boys had muscle and epithelial cells seeded onto tubularized polyglycolic acid:poly (lactide-co-glycolide acid) scaffolds. Patients then underwent urethral reconstruction of the tubularized urethras. At a median follow-up of 71 months, all patients had a patent urethra (52).
Although there has been a tremendous amount of basic science and animal research for tissue engineered grafts, few platforms have advanced to human clinical trial. The problem for any graft in treating a complex urethral stricture is related to the issues of ischemia, fibrosis, and wound contracture. Further study is needed to better elucidate the optimal scaffolds and cell sources to overcome the inherent issues of tissue fibrosis associated with complex urethral strictures.
Conclusions
Anti-fibrotic injectables are an intriguing attempt at improving endoscopic stricture management. While preliminary data is encouraging, more rigid outcomes measures will be critical to properly evaluate the role these adjuncts play with regards to traditional treatment. Well-controlled clinical trials with long-term follow-up are needed to determine the optimal antifibrotic drug and technical approach for these novel agents.
Urethral reconstruction continues to be an evolving specialty due to the uniform difficulty in treating complex urethral strictures. The reconstructive urologist needs to be comfortable with a number of grafts in order to best treat each individual patient’s condition. Tissue engineered grafts continue to show promise, but further study is needed to improve upon existing platforms.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Simman R, Alani H, Williams F. Effect of mitomycin C on keloid fibroblasts: an in vitro study. Ann Plast Surg 2003;50:71-6. [PubMed]
- Ferguson B, Gray SD, Thibeault S. Time and dose effects of mitomycin C on extracellular matrix fibroblasts and proteins. Laryngoscope 2005;115:110-5. [PubMed]
- Ayyildiz A, Nuhoglu B, Gülerkaya B, et al. Effect of intraurethral Mitomycin-C on healing and fibrosis in rats with experimentally induced urethral stricture. Int J Urol 2004;11:1122-6. [PubMed]
- Ubell ML, Ettema SL, Toohill RJ, et al. Mitomycin-c application in airway stenosis surgery: analysis of safety and costs. Otolaryngol Head Neck Surg 2006;134:403-6. [PubMed]
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009;119:272-83. [PubMed]
- Simpson CB, James JC. The efficacy of mitomycin-C in the treatment of laryngotracheal stenosis. Laryngoscope 2006;116:1923-5. [PubMed]
- Reibaldi A, Uva MG, Longo A. Nine-year follow-up of trabeculectomy with or without low-dosage mitomycin-c in primary open-angle glaucoma. Br J Ophthalmol 2008;92:1666-70. [PubMed]
- Murakami M, Mori S, Kunitomo N. Studies on the pterygium. V. Follow-up information of mitomycin C treatment. Nihon Ganka Gakkai Zasshi 1967;71:351-8. [PubMed]
- Mueller CM, Beaunoyer M, St-Vil D. Topical mitomycin-C for the treatment of anal stricture. J Pediatr Surg 2010;45:241-4. [PubMed]
- Dolmetsch AM. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology 2010;117:1037-40. [PubMed]
- Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology 2002;109:1336-41; discussion 1341-2. [PubMed]
- Betalli P, De Corti F, Minucci D, et al. Successful topical treatment with mitomycin-C in a female with post-brachytherapy vaginal stricture. Pediatr Blood Cancer 2008;51:550-2. [PubMed]
- Wolfram D, Tzankov A, Pülzl P, et al. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 2009;35:171-81. [PubMed]
- Poynter JH, Levy J. Balanitis xerotica obliterans: effective treatment with topical and sublesional corticosteroids. Br J Urol 1967;39:420-5. [PubMed]
- Hebert PW. The treatment of urethral stricture: transurethral injection of triamcinolone. J Urol 1972;108:745-7. [PubMed]
- Tavakkoli Tabassi K, Yarmohamadi A, Mohammadi S. Triamcinolone injection following internal urethrotomy for treatment of urethral stricture. Urol J 2011;8:132-6. [PubMed]
- Kumar S, Kapoor A, Ganesamoni R, et al. Efficacy of holmium laser urethrotomy in combination with intralesional triamcinolone in the treatment of anterior urethral stricture. Korean J Urol 2012;53:614-8. [PubMed]
- Mazdak H, Izadpanahi MH, Ghalamkari A, et al. Internal urethrotomy and intraurethral submucosal injection of triamcinolone in short bulbar urethral strictures. Int Urol Nephrol 2010;42:565-8. [PubMed]
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol 2007;51:1089-92; discussion 1092. [PubMed]
- Chung JH, Kang DH, Choi HY, et al. The effects of hyaluronic acid and carboxymethylcellulose in preventing recurrence of urethral stricture after endoscopic internal urethrotomy: a multicenter, randomized controlled, single-blinded study. J Endourol 2013;27:756-62. [PubMed]
- Kumar S, Garg N, Singh SK, et al. Efficacy of Optical Internal Urethrotomy and Intralesional Injection of Vatsala-Santosh PGI Tri-Inject (Triamcinolone, Mitomycin C, and Hyaluronidase) in the Treatment of Anterior Urethral Stricture. Adv Urol 2014;2014:192710.
- Wessells H, McAninch JW. Current controversies in anterior urethral stricture repair: free-graft versus pedicled skin-flap reconstruction. World J Urol 1998;16:175-80. [PubMed]
- Dubey D, Vijjan V, Kapoor R, et al. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol 2007;178:2466-9. [PubMed]
- Palminteri E, Brandes SB, Djordjevic M. Urethral reconstruction in lichen sclerosus. Curr Opin Urol 2012;22:478-83. [PubMed]
- Dubey D, Sehgal A, Srivastava A, et al. Buccal mucosal urethroplasty for balanitis xerotica obliterans related urethral strictures: the outcome of 1 and 2-stage techniques. J Urol 2005;173:463-6. [PubMed]
- Simonato A, Gregori A, Ambruosi C, et al. Lingual mucosal graft urethroplasty for anterior urethral reconstruction. Eur Urol 2008;54:79-85. [PubMed]
- Barbagli G, De Angelis M, Romano G, et al. The use of lingual mucosal graft in adult anterior urethroplasty: surgical steps and short-term outcome. Eur Urol 2008;54:671-6. [PubMed]
- Xu YM, Fu Q, Sa YL, et al. Treatment of urethral strictures using lingual mucosas urethroplasty: experience of 92 cases. Chin Med J (Engl) 2010;123:458-62. [PubMed]
- Das SK, Kumar A, Sharma GK, et al. Lingual mucosal graft urethroplasty for anterior urethral strictures. Urology 2009;73:105-8. [PubMed]
- Sharma GK, Pandey A, Bansal H, et al. Dorsal onlay lingual mucosal graft urethroplasty for urethral strictures in women. BJU Int 2010;105:1309-12. [PubMed]
- Sharma AK, Chandrashekar R, Keshavamurthy R, et al. Lingual versus buccal mucosa graft urethroplasty for anterior urethral stricture: a prospective comparative analysis. Int J Urol 2013;20:1199-203. [PubMed]
- Xu YM, Feng C, Sa YL, et al. Outcome of 1-stage urethroplasty using oral mucosal grafts for the treatment of urethral strictures associated with genital lichen sclerosus. Urology 2014;83:232-6. [PubMed]
- Simonato A, Gregori A. Oral complications after lingual mucosal graft harvest for urethroplasty. ANZ J Surg 2008;78:933-4. [PubMed]
- Kumar A, Goyal NK, Das SK, et al. Oral complications after lingual mucosal graft harvest for urethroplasty. ANZ J Surg 2007;77:970-3. [PubMed]
- Presman D, Greenfield DL. Reconstruction of the perineal urethra with a free full-thickness skin graft from the prepuce. J Urol 1953;69:677-80. [PubMed]
- Devine PC, Horton CE, Devine CJ Sr, et al. Use of full thickness skin grafts in repair of urethral strictures. J Urol 1963;90:67-71. [PubMed]
- Devine CJ Jr, Horton CE. A one stage hypospadias repair. J Urol 1961;85:166-72. [PubMed]
- Lumen N, Oosterlinck W, Hoebeke P. Urethral reconstruction using buccal mucosa or penile skin grafts: systematic review and meta-analysis. Urol Int 2012;89:387-94. [PubMed]
- Barbagli G, Kulkarni SB, Fossati N, et al. Long-term followup and deterioration rate of anterior substitution urethroplasty. J Urol 2014;192:808-13. [PubMed]
- Manoj B, Sanjeev N, Pandurang PN, et al. Postauricular skin as an alternative to oral mucosa for anterior onlay graft urethroplasty: a preliminary experience in patients with oral mucosa changes. Urology 2009;74:345-8. [PubMed]
- Chen ML, Odom BD, Johnson LJ, et al. Combining ventral buccal mucosal graft onlay and dorsal full thickness skin graft inlay decreases failure rates in long bulbar strictures (≥6 cm). Urology 2013;81:899-902. [PubMed]
- Schreiter F, Noll F. Mesh graft urethroplasty using split thickness skin graft or foreskin. J Urol 1989;142:1223-6. [PubMed]
- Kessler TM, Schreiter F, Kralidis G, et al. Long-term results of surgery for urethral stricture: a statistical analysis. J Urol 2003;170:840-4. [PubMed]
- Pfalzgraf D, Olianas R, Schreiter F, et al. Two-staged urethroplasty: buccal mucosa and mesh graft techniques. Aktuelle Urol 2010;41 Suppl 1:S5-9. [PubMed]
- Kinkead TM, Borzi PA, Duffy PG, et al. Long-term followup of bladder mucosa graft for male urethral reconstruction. J Urol 1994;151:1056-8. [PubMed]
- Mundy AR, Andrich DE. Entero-urethroplasty for the salvage of bulbo-membranous stricture disease or trauma. BJU Int 2010;105:1716-20. [PubMed]
- Xu YM, Qiao Y, Sa YL, et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol 2009;182:1040-3. [PubMed]
- Fiala R, Vidlar A, Vrtal R, et al. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol 2007;51:1702-8; discussion 1708.
- Palminteri E, Berdondini E, Colombo F, et al. Small intestinal submucosa (SIS) graft urethroplasty: short-term results. Eur Urol 2007;51:1695-701; discussion 1701.
- Palminteri E, Berdondini E, Fusco F, et al. Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 2012;79:695-701. [PubMed]
- Bhargava S, Patterson JM, Inman RD, et al. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol 2008;53:1263-9. [PubMed]
- Raya-Rivera A, Esquiliano DR, Yoo JJ, et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 2011;377:1175-82. [PubMed]