
Cause-specific mortality of low and selective intermediate-risk prostate cancer patients with active surveillance or watchful waiting
Introduction
Prostate cancer (PCa) remains the most common male cancer and is among the leading cause of cancer-related deaths in men in industrialized countries. In 2017, approximately 1.3 million men were diagnosed with PCa worldwide and there were 416,000 associated deaths (1). Due to the widespread use of PSA screening, the mortality rate of the disease has declined by more than 50% (2). The results of the European Randomized Study of Screening Prostate Cancer revealed that a 20% reduction in mortality was attributable to PSA screening and treatment; however, 48 men had to be overtreated to prevent 1 cause-specific death from prostate cancer (3). Almost 60% of men who are diagnosed with prostate cancer may not require active therapy (4).
Active surveillance or watchful waiting (AS/WW) is an alternative to radical prostatectomy or radiotherapy, and for appropriately selected patients AS/WW can reduce overtreatment (5). The 2019 National Comprehensive Cancer Network (NCCN) Guidelines suggested that observation may be an option for men with low-risk or favorable intermediate-risk PCa (no more than 1 intermediate risk factor, ISUP grade ≤2, and <50% of biopsy cores positive) (6). A growing subset of prostate cancer patients are recognized to be candidates for AS/WW and AS/WW is increasingly being used (7-9). Nevertheless, the outcomes of observation in men with favorable intermediate-risk PCa are unclear and have produced mixed results (10,11). A nomogram to guide the clinical selection of PCa patients who are suitable for AS/WW has yet to be developed.
To improve the prediction of prognosis for patients with ISUP grade 1 or 2 PCa who conform to the NCCN definition of low-risk PCa [prostate specific antigen (PSA) <10 ng/mL and cT2aN0M0 or less], we reviewed the information of patients registered in the Surveillance, Epidemiology, and End Results (SEER) Program from 2004 to 2015 and analyzed the association of tumor characteristics with prostate cancer-specific mortality (PCSM) and non-PCSM (considered a competing risk). We also constructed and validated a competing-risk nomogram to predict PCSM in order to assist clinical decision-making for PCa patients. We present the following article in accordance with the TRIPOD reporting checklist (available at http://dx.doi.org/10.21037/tau-20-994).
Methods
Study patients
Data of PCa cases diagnosed between 2004 to 2015 were extracted from the SEER Incidence database using the SEER*Stat software (version 8.3.5) (https://seer.cancer.gov/seerstat/software/). The inclusion criteria for cases were low-risk (LR) (ISUP =1 and PSA <10 ng/mL and cT1-2aN0M0) and selective intermediate-risk (SIR) (ISUP =2 and PSA <10 ng/mL and cT1-2aN0M0) patients with AS/WW. The exclusion criteria included: (I) incomplete clinical data; (II) patients with >1 primary cancer; (III) patients who received intervention treatments such as transurethral resection of the prostate, radical prostatectomy, radiotherapy, or chemotherapy; (IV) patients who were recommended treatment but refused it; (V) patients with uncertain cause of death. Prostate cancer-specific mortality was defined as death as a result of prostate. Competing mortality was defined as either non-prostate cancer mortality. For further analysis, age and PSA were used as continuous variables. Marital status was categorized as married or unmarried. Race was classified into white, black, or other. The clinical primary tumor category extension (cT) categories of the cases diagnosed between 2004–2009 were converted according to the American Joint Committee on Cancer (AJCC) 7th edition [available on the SEER Registrar Staging Assistant website; https://staging.seer.cancer.gov/cs/input/02.05.50/prostate/extension/?breadcrumbs=(~schema_list~),(~view_schema~,~prostate~)], in line with the cases between 2010–2015.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The data analyzed in this study are freely available from the SEER Incidence database (https://seer.cancer.gov/) and required no ethical approval.
Statistical analysis
The median follow-up values were the median observed survival time for cases between 2004–2015. For local PCa cases that had a low risk of clinical progression within 10–15 years of diagnosis (12), the prognosis more vulnerable to competing events with increasing age, such as cardiovascular and cerebrovascular diseases. Therefore, the causes of death were divided into PCSM and non-PCSM, and the competing risk model was used for the analysis (13). The cumulative incidence function (CIF) was used to show the PCSM and non-PCSM for all patients and Gray’s test was used to evaluate the difference (14). The PCSM and non-PCSM at 5 and 10 years were predicted with the Fine-Gray proportional hazards regression model. Subsequently, the cases were randomly divided into the training or validation cohort (1:1). A nomogram was constructed in the training cohort and validated using the validation cohort to visualize the competing risk models (15). Variables, including cT stage, race, age, marital status, PSA, and ISUP grade, which were significantly associated with outcomes were incorporated into the final nomogram. Finally, the performance of the model was evaluated through discrimination and calibration (16). Discrimination was defined to as the model’s ability to identify events and was evaluated using the concordance index (C-index). The calibration curve was used to evaluate the agreement between the predictions of the model and observations using 500 bootstrap resamples.
All statistical analyses were performed using R software (version 3.6.1 software, https://www.r-project.org). The R packages “cmprsk”, “rms”, “mstate”, and “pec” were used to model and develop the nomogram. A two-sided P value of <0.05 was considered to be statistically significant.
Results
PCSM and competing risk analysis
A total of 30,538 patients diagnosed from 2004 to 2015 on the SEER database were eligible for inclusion in our analysis. The detailed demographics and tumor characteristics of these patients are summarized in Table 1.
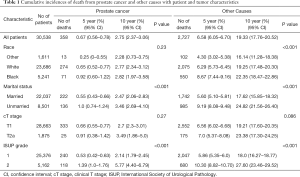
Full table
The median follow-up of all patients was 60 months (range, 1–155 months). Among the 30,538 cases, there were 358 (1.17%) deaths resulting from PCa and 2,727 (8.93%) resulting from other causes. The 10-year cumulative incidence of death from prostate cancer and death from other cause were 2.8% (95% CI: 2.4–3.1%) and 19.3% (95% CI: 17.8–20.5%), respectively (Figure 1A). Among the other causes of death, the three most common causes were cardiac diseases (31.9%), chronic obstructive pulmonary disease and associated conditions (8.6%), and cerebrovascular disease (7.0%). Table 1 summarizes the 5- and 10-year PCSM and non-PCSM, together with patient and tumor characteristics. Figure 1 demonstrates the corresponding CIF curves.
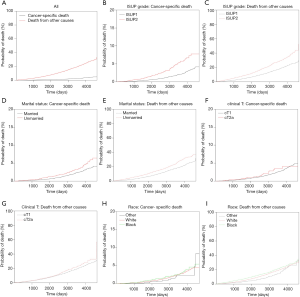
Subsequently, competing risk analysis was performed to define whether the variables could predict the PCSM and non-PCSM. Statistically significant variables associated with PCSM in this model included age (P<0.001), marital status (P<0.001), PSA (P<0.001), and ISUP grade (P<0.001). PCSM was higher among older patients, with a sub-distribution hazard ratio (sdHR) of 1.07 (95% CI: 1.05–1.08). Every extra unit of PSA was associated with a significant increase in PCSM, with an sdHR of 1.10 (95% CI: 1.04–1.20). Patients with ISUP 2 were more likely to die of PCa (sdHR =1.89, 95% CI: 1.51–2.37). Unmarried patients had a higher PCSM than those who were married (sdHR =1.61, 95% CI: 1.29–2.0). Black patients had higher PCSM than patients who were white or of other races, but there were no significant differences. Patients with cT2a had a higher PCSM than cT1 patients, but there were no significant differences. Similarly, older age, unmarried status, white and black race, higher PSA, and higher ISUP grade increased non-PCSM; however, cT2a patients had higher non-PCSM than cT1 patients, although there were no significant differences (Table 2).
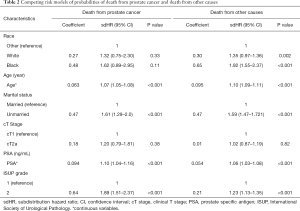
Full table
PCSM nomogram
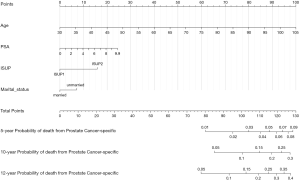
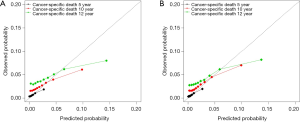
All patients were randomly divided into the training or the validation cohort (1:1). The detailed demographics and tumor characteristics of the two cohorts are summarized in Table S1. A nomogram to predict the probability of PCSM at 5, 10, and 12 years was constructed in the training cohort, based on age, PSA, marital status, and ISUP grade (Figure 2). The nomogram had a reliable performance in predicting PCSM, with a C-index of 0.744 (95% CI: 0.700–0.781, P<0.001). The calibration curve based on the training cohort showed good agreement between prediction and observation in 5-, 10-, and 12-year PCSM (Figure 3A) . Similarly, in the validation cohort, the nomogram indicated excellent accuracy in predicting PCSM, with a C-index of 0.738 (95% CI: 0.700–0.777, P<0.001). The calibration curve also showed that the nomogram had an excellent performance in predicting PCSM in the validation cohort (Figure 3B).
Discussion
The challenges associated with the application of PSA have emphasized the need to develop more objective measures for identifying clinically significant PCa while continuing to reduce the sequence of over-diagnosis and over-treatment (17). In randomized controlled trials, AS/WW has been proven as an alternative clinical strategy to active treatment for appropriately selected patients (18,19). A number of research about AS/WW have reported favorable short- to medium- term outcomes among low-risk PCa patients, including cohorts from Johns Hopkins University (9), Memorial Sloan-Kettering Cancer Center (20), the Royal Marsden Hospital (21), University of California, San Francisco (22,23), and the University of Toronto (24).
However, most studies to date have focused on AS/WW for low-risk or intermediate-risk PCa patients whose life expectancy is less than 10 years. Moreover, the prognoses of intermediate-risk PCa patients with AS/WW are complex and varied (18,25) due to different inclusion criteria and the lack of a reliable PCSM Nomogram. The University of Toronto cohort included a total of 450 patients with AS, 14% with PSA higher than 10 ng/mL, 17% with ISUP2 and ISUP3, and 3%with both risk factors (24). However, the results did not accurately identify high-risk patients who were not suitable for AS. In the study of Cooperberg et al., PCa patients with AS were classified as low- or intermediate-rise based on the UCSF Cancer of the Prostate Risk Assessment (CAPRA) score. And they found that part of intermediate-risk patients may be appropriate candidates for AS (23). Similarly, Musunuru et al. included LR patients and part of intermediate-risk patients (age >70 years and cT2c or PSA ≤15 ng/mL). Their results showed that LR and intermediate-risk patients with ISUP1 could receive AS, but not for ISUP2 PCa (26). Ploussard et al. also tried to explore the inclusion criteria of AS and conducted a retrospective analysis including 2,323 patients with localized ISUP2 PCa. Their research suggests that patients with ISUP2 PCa could receive AS but should adhere to strict selection criteria (10). Thus, development of a nomogram may be especially beneficial for select which patients to receive AS.
Further, the 2019 NCCN Guidelines recommend AS/WW as an option for patients with favorable intermediate-risk PCa (no more than 1 intermediate risk factor, ISUP grade ≤2, and <50% of biopsy cores positive) (6). Therefore, we included LR and SIR PCa patients with AS/WW in our study to predict their prognosis and to provide some guidance for clinical trials. A nomogram to predict PCSM of LR and SIR patients with AS/WW was constructed and validated. To our knowledge, the current study is the first report of AS/WW use for patients with LR and SIR prostate cancer to be based on a large population-based database across the United States. Therefore, the nomogram we designed is the first to be used to identify patients potentially suitable for AS/WW and for the design of clinical trials involving intermediate risk patients with AS/WW.
We found that patients with SIR ISUP2 had higher PCSM than those with LR ISUP1 after adjusting for PSA, cT stage, race, and marital status. This result is consistent with previous findings that SIR PCa patients with AS are more likely than LR patients to upgrade to unfavorable disease (27). In addition, Raldow et al. found that favorable intermediate risk PCa did not have significantly increased risk of PCSM compared with low-risk PCa following radiotherapy and ISUP grade did not have statistical significance for PCSM (28). It may reveal that favorable intermediate risk PCa could benefit from radiotherapy. However, Butler et al. reported that PCSM of favorable intermediate risk PCa have no statistical difference between radical prostatectomy/radiotherapy and AS/WW (28). To sum up, it implies that AS/WW treatment for SIR patients is feasible, but should be followed more closely. Furthermore, most evidence for the application of AS/WW in SIR patients has come from retrospective data. Therefore, prospective trials are needed to further evaluate the safety of AS/WW for SIR patients.
Between 2010 and 2015, the number of black and non-black patients with AS/WW increased (29). Our findings show that the difference in PCSM between black and white patients was not statistically significant. Previous studies based on the SEER database from 2010–2015 show that PCSM was significantly higher for black patients with low-grade ISUP1 who underwent AS/WW than their non-black counterparts (30); however, the median follow-up in Mahal et al.’s study was 36 months, which was shorter than our study’s median follow-up time of 60 months. Therefore, we infered that racial disparities might not exist among patients who receive AS/WW.
In accordance with previous studies, our data also showed that high PSA was associated with an increased risk of PCSM among PCa patients with AS/WW (26,31). Furthermore, patients who were unmarried had higher risks of PCSM and non-PCSM than those who were married, and this finding is supported by multiple retrospective reviews (32,33). Overall, our nomogram was consistent with the results of previous studies, indicating its reliability and helpfulness in predicting the prognosis of PCa with AS/WW.
We noted that older age had a negative impact on PCSM in patients with AS/WW (sdHR =1.07, 95% CI: 1.05–1.08), and the impact on non-PCSM was even more significant (sdHR =1.10, 95% CI: 1.09–1.11), which was consistent with the findings of previously published literature (33-35). This result implies that with increasing age, non-PCSM becomes higher than that of PCSM. Therefore, the possibility of non-PCSM should be taken into consideration during clinical decision-making for elderly patients. Younger patients are therefore suitable for AS/WW as they have lower PCSM, but need longer follow-up times.
Our study has several limitations. Firstly, the analyses of data were retrospective and heterogeneous. Secondly, the SEER database cannot discriminate between AS and WW. Thirdly, the SEER database lacks information, such as comorbidities, subsequent treatments, and the percentage of Gleason pattern 4 in specimens. Additionally, the SEER database does not contain information on the number of positive cores, which is an important inclusion criterion for AS/WW. We believe that our inclusion criteria (ISUP ≤2 and PSA <10 ng/mL and cT1-2aN0M0) are by and large opposite to the concept of clinically significant disease based on expression of “definition one” (36). It also conforms to the criteria of favorable intermediate-risk PCa defined in the 2019 NCCN Guidelines (6).
In conclusion, the prognosis of LR and SIR PCa patients with AS/WW was excellent. Our competing risk nomogram showed a good performance in predicting PCSM. It could serve as a useful clinical tool for identifying patients with higher risk of PCSM and selecting candidates for AS/WW.
Acknowledgments
Funding: This study was supported by grants from National Natural Science Foundation of China (No.81600542 and 81670643); the Natural Science Foundation of Guangdong Province (2020A1515010464); the Guangdong Basic and Applied Basic Research Foundation (Grant NO. 2019A1515110033); Distinguished Young Talents in Higher Education Foundation of Guangdong Province (Grant NO. 2019KQNCX115 and 2020KZDZX1168); China Postdoctoral Science Foundation (Grant NO. 2019M662865); Achievement cultivation and clinical transformation application cultivation projects of the First Affiliated Hospital of Guangzhou Medical University (Grant NO. ZH201908).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-994
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-994). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The data analyzed in this study are freely available from the SEER Incidence database (https://seer.cancer.gov/) and required no ethical approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fitzmaurice C, Abate D, Abbasi N, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Catalona WJ. Prostate Cancer Screening. Med Clin North Am 2018;102:199-214. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605-13. [Crossref] [PubMed]
- Kinsella N, Stattin P, Cahill D, et al. Factors Influencing Men's Choice of and Adherence to Active Surveillance for Low-risk Prostate Cancer: A Mixed-method Systematic Review. Eur Urol 2018;74:261-80. [Crossref] [PubMed]
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:479-505. [Crossref] [PubMed]
- Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol 2015;67:44-50. [Crossref] [PubMed]
- Mahal BA, Butler S, Franco I, et al. Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010-2015. JAMA 2019;321:704-6. [Crossref] [PubMed]
- Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol 2002;167:1231-4. [Crossref] [PubMed]
- Ploussard G, Isbarn H, Briganti A, et al. Can we expand active surveillance criteria to include biopsy Gleason 3+4 prostate cancer? A multi-institutional study of 2,323 patients. Urol Oncol 2015;33:71.e1-9. [Crossref] [PubMed]
- Yamamoto T, Musunuru HB, Vesprini D, et al. Metastatic Prostate Cancer in Men Initially Treated with Active Surveillance. J Urol 2016;195:1409-14. [Crossref] [PubMed]
- Thomsen FB, Brasso K, Klotz LH, et al. Active surveillance for clinically localized prostate cancer--a systematic review. J Surg Oncol 2014;109:830-5. [Crossref] [PubMed]
- Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133:601-9. [Crossref] [PubMed]
- Wolbers M, Koller MT, Stel VS, et al. Competing risks analyses: objectives and approaches. Eur Heart J 2014;35:2936-41. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. [Crossref] [PubMed]
- Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol 2004;171:1520-4. [Crossref] [PubMed]
- Ulmert D, Serio AM, O'Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol 2008;26:835-41. [Crossref] [PubMed]
- Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008;112:2664-70. [Crossref] [PubMed]
- Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol 2011;29:228-34. [Crossref] [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [Crossref] [PubMed]
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932-42. [Crossref] [PubMed]
- Musunuru HB, Yamamoto T, Klotz L, et al. Active Surveillance for Intermediate Risk Prostate Cancer: Survival Outcomes in the Sunnybrook Experience. J Urol 2016;196:1651-8. [Crossref] [PubMed]
- Gearman DJ, Morlacco A, Cheville JC, et al. Comparison of Pathological and Oncologic Outcomes of Favorable Risk Gleason Score 3 + 4 and Low Risk Gleason Score 6 Prostate Cancer: Considerations for Active Surveillance. J Urol 2018;199:1188-95. [Crossref] [PubMed]
- Raldow AC, Zhang D, Chen MH, et al. Risk Group and Death From Prostate Cancer: Implications for Active Surveillance in Men With Favorable Intermediate-Risk Prostate Cancer. JAMA Oncol 2015;1:334-40. [Crossref] [PubMed]
- Butler S, Muralidhar V, Chavez J, et al. Active Surveillance for Low-Risk Prostate Cancer in Black Patients. N Engl J Med 2019;380:2070-2. [Crossref] [PubMed]
- Mahal BA, Berman RA, Taplin ME, et al. Prostate Cancer-Specific Mortality Across Gleason Scores in Black vs Nonblack Men. JAMA 2018;320:2479-81. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Loeb S, Byrne N, Makarov DV, et al. Use of Conservative Management for Low-Risk Prostate Cancer in the Veterans Affairs Integrated Health Care System From 2005-2015. JAMA 2018;319:2231-3. [Crossref] [PubMed]
- Knipper S, Pecoraro A, Palumbo C, et al. A 25-year Period Analysis of Other-cause Mortality in Localized Prostate Cancer. Clin Genitourin Cancer 2019;17:395-401. [Crossref] [PubMed]
- Butler SS, Mahal BA, Lamba N, et al. Use and early mortality outcomes of active surveillance in patients with intermediate-risk prostate cancer. Cancer 2019;125:3164-71. [Crossref] [PubMed]
- Abdollah F, Sun M, Schmitges J, et al. Cancer-specific and other-cause mortality after radical prostatectomy versus observation in patients with prostate cancer: competing-risks analysis of a large North American population-based cohort. Eur Urol 2011;60:920-30. [Crossref] [PubMed]
- Simmons LAM, Ahmed HU, Moore CM, et al. The PICTURE study -- prostate imaging (multi-parametric MRI and Prostate HistoScanning™) compared to transperineal ultrasound guided biopsy for significant prostate cancer risk evaluation. Contemp Clin Trials 2014;37:69-83. [Crossref] [PubMed]


