
Phosphatidylcholine and L-acetyl-carnitine-based freezing medium can replace egg yolk and preserves human sperm function
Introduction
Developments in assisted reproductive technologies (ART) have evolved rapidly over the past decades and seminal freezing has become an integral aspect of reproductive medicine (1). The preservation of semen in a therapeutic program is fundamental in procedures that can lead to testicular insufficiency or ejaculatory dysfunction, such as radiotherapy, chemotherapy and invasive surgeries (2,3). It is also indicated in ART in cases of donor insemination, vasectomy, and surgically retrieved spermatozoa to avoid the need to repeat invasive surgical procedures and ensure the presence of viable cells on the day of follicular aspiration (4).
Although long-term sperm storage is one of the most important tools to improve ART, cryopreservation of semen results in a significant increase in the percentage of poorly motile sperm or sperm with both abnormal morphology and DNA integrity, which impairs their ability to fertilize (5-8). In addition, spermatozoa with low motility from asthenozoospermic men are particularly susceptible to damages induced by cryopreservation and decreased fertilizing ability post-thawing (9). Conventional cryopreservation methods induce chemical and mechanical damage to the sperm membranes due to changes in lipid phase transition and increased lipid peroxidation (2,10), associated with loss of functional competence (11) and sperm DNA fragmentation (12,13).
Different strategies have been developed to avoid sperm damage during the cryopreservation process (14-17). Currently, freezing media based on egg yolk are the most used in ART, and its action is due to the egg yolk being a concentrate of phospholipids, lipoproteins and cholesterol, which helps to restore lost lipids of the sperm membrane during the processes of freezing and thawing (18). The cryoprotectant potential of phospholipids of vegetal origin as soybean lecithin has been investigated as a substitute for egg yolk in diluents used for the cryopreservation of human spermatozoa (19-21). The non-animal origin and the minimum health risks associated with the use of soybean lecithin make its application preferable and, in terms of biosafety, can be considered superior to egg yolk (22).
The International Lecithin and Phospholipid Society defines lecithin as a complex mixture of glycerophospholipids obtained from animal, vegetable or microbial sources, containing varying amounts of triglycerides, fatty acids, glycolipids, sterols, and sphingophospholipids. There are different chemical species of phosphatidylcholine (PC) derived from soybean and egg sources, with different chemical chain length and degree of unsaturation (23). We have already shown previously that purified soybean PC (Lα-phosphatidylcholine >99%) has cryoprotective effects similar to egg yolk when used as an additive for human sperm freezing medium (24,25). However, the efficacy of purified soy-PC in preserving the mitochondrial function and cardiolipin content of spermatozoa during cryopreservation of ovine semen was described as inferior to the egg yolk, which may be associated with the loss of antioxidants in soybean PC-based freezing medium (26).
L-acetyl-carnitine, a quaternary amine (3-hydroxy-4-N-trimethylamino-butyrate) synthesized from the essential amino acids lysine and methionine, is an antioxidant used in the treatment of men with low seminal quality, since carnitines remove the toxic excess of intracellular acetyl-CoA, protecting the spermatozoa from oxidative damage (27-29). In addition, carnitines play a key role in cell energy generation, transferring free fatty acids from the cytosol to mitochondria, facilitating their oxidation and generation of adenosine triphosphate (27,30). Due to its antioxidant properties, protective of both cell oxidative metabolism and mitochondrial membrane, L-acetyl-carnitine was incorporated into the soyPC-based cryoprotectant formulation (24,31).
The new freezing medium (ANTIOX-PC) was designed from a basal medium containing salts, carbohydrates, purified proteins, amino acids, and synthetic cholesterol. To the basal medium were added the antioxidant L-acetyl-carnitine and a high purity synthetic soybean PC (INVITRA—Assisted Reproduction Technologies, Brazil). Therefore, the objective of this study was comparing the ANTIOX-PC with the conventional cryoprotectant Test Yolk Buffer (TYB—Irvine Scientific) regarding post-thaw seminal quality and the DNA integrity outcomes in semen from both men with normal or low motility, in order to evaluate if the phospholipid and antioxidant supplementation from ANTIOX-PC can be used in the replacement of cryoprotective medium containing egg yolk. We present the following article in accordance with the TREND reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1004).
Methods
Subjects and semen samples
This is a prospective experimental study that was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Local Research Ethics Committee and also by the National Commission for Ethics in Research (Protocols: CAAE 44677215.0.0000.5440 and CAAE 35850214.4.0000.5440) and informed consent was taken from all the patients. The volunteers were recruited at the Human Reproduction Sector of the Clinical Hospital of the Ribeirao Preto Medical School, University of São Paulo (HCFMRP/USP), where they sought to investigate conjugal infertility; and at the Urology Outpatient Clinic of the State Hospital of Ribeirao Preto “Prof. Dr. Carlos Eduardo Martinelli”, where they came for a birth control consultation for possible vasectomy surgery.
Semen analysis
Ejaculates were obtained from 121 men from June 2015 to March 2018. All semen samples were obtained on-site by masturbation into sterile containers after at least 72 h of ejaculatory abstinence and left to liquefy at 37 °C on a tube warmer for 30 minutes. Basic semen analysis was performed in the andrology laboratory of HCFMRP/USP within 1 hour of collection and comprised the measurements of semen volume and sperm concentration, motility, vitality, and morphology. All the parameters were measured in accordance with WHO guidelines (32). The sperm morphology was evaluated according to Krüger’s criteria (33) and the sperm vitality was assessed by using eosin-nigrosin test (32). Only samples showing sperm count of ≥15×106/mL and minimum volume of 1 mL were included in this study. The motility of each sperm sample was graded in four clusters: progressive motility, nonprogressive motility, non-motility cells, and total motility, which were reported as percentages. The samples were divided into two groups according to progressive motility: (I) samples with progressive motility ≥32% (normal motility) (n=63); (II) samples with progressive motility <32% (low motility) (n=58).
Experimental design
Following the routine semen analysis, each semen sample was divided into two aliquots, and 0.5 mL of the fresh raw semen was pipetted into cryovials (Nalge Company, Rochester, NY) and mixed 1:1 with one of two freezing media: (I) commercially available Freezing Medium TEST Yolk Buffer (TYB) (Irvine Scientific, Santa Ana, CA, USA); (II) synthetic freezing medium supplemented with soybean PC and L-acetyl-carnitine (ANTIOX-PC) (INVITRA—Assisted Reproduction Technologies, Brazil). Therefore, semen and cryoprotectants were combined to a final 1:1 (v/v) reaching a final volume of 1 mL. First, all cryotubes were immersed in cold water at 4 °C and refrigerated for 30 minutes. Subsequently, the cryotubes were suspended in liquid nitrogen vapor (5 cm above the level of liquid nitrogen, −80 °C) for 10 minutes. Finally, the aliquots were plunged into liquid nitrogen and stored at −196 °C. Thawing step was performed during 30 minutes at room temperature. After centrifugation at 400 ×g for 5 minutes, the pellet was resuspended in 1 mL of Human Tubal Fluid (HTF) (Irvine Scientific) supplemented with 10% Synthetic Serum Substitute (SSS) (Irvine Scientific). Samples were randomly thawed at least 30 days after freezing and analyzed with regard to the percentage of motile sperm cells, vitality, morphology, and DNA integrity by TUNEL assay. Two experimental repetitions were performed during routine semen analysis and the sperm DNA fragmentation index evaluation to avoid technical variation. The sample size calculation was performed using an online software (https://clincalc.com/stats/samplesize.aspx), and it was determined that it would be necessary to evaluate at least 53 cases per group (total of 106 subjects) for have a security of 80%, alpha 2.5%.
Preparation of cryoprotective medium
The ANTIOX-PC medium was designed and produced in small scale for research by INVITRA®, based on the standard buffer composition with modifications including the addition of synthetic human serum albumin, synthetic cholesterol, L-α-phosphatidylcholine and L-acetyl-carnitine. Unless stated otherwise, all of the chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Basic freezing medium
The basic freezing media consisted of a Zwitter ion buffer system (TEST buffer solution) prepared in-house by diluting 4.325 g TES (N-tris-hydroxy methyl 2 aminoethane sulfonic acid), 1.0269 g TRIS (hydroxy methyl aminomethane-trizma base), 1 g dextrose, 0.015 g penicillin G, and 0.025 g streptomycin sulphate to 100 mL with HPLC grade water (Fisher Scientific, Pittsburgh, PA, USA). To the second dilution (TES-TRIS-CITRATE buffer solution) 68% (v/v) TEST (TES-TRIS) 30% (v/v), 325 mOsm sodium citrate pH 7; 0,2% (v/v) 325 mOsm fructose were mixed and centrifuged at 10,000 ×g for 20 minutes and the solution was filtered through 0.22 µ sterile filter. To final TEST freezing medium formulation (pH 7.4) were added 10% (v/v) glycerol, 2% (v/v) dimethyl sulfoxide (DMSO), 2% (w/v) human serum albumin, and synthetic cholesterol (25).
Lα-phosphatidylcholine and L-carnitine-based medium
Lα-phosphatidylcholine and L-acetyl carnitine were added to the basic freezing medium at 3% and 6% w/v, respectively. Briefly, the PC and L-carnitine supplements were previously diluted and added to the BFM. Immediately, the cryoprotectant medium was gently homogenized and stored in 0.5 mL-aliquots at −20 °C (25,31).
Test yolk freezing medium
This commercially purchased freezing medium (Irvine Scientific) was used as a control egg yolk medium.
DNA fragmentation index
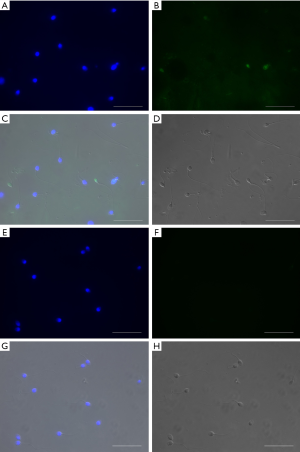
Post-thawed spermatozoa were washed in Human Tubal Fluid (HTF) (Irvine Scientific) supplemented with 10% Synthetic Serum Substitute (SSS) (Irvine Scientific) and then with Phosphate Buffered Saline (PBS) 1× (Sigma-Aldrich) and centrifuged at 500 ×g for 5 min. After that, sperm samples were washed and resuspended in PBS solution at a final concentration of 20×106/mL; and fixed in methanol 80%. Then slides were washed and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich). Terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL) (In situ cell death detection kit with fluorescein, Roche Diagnostics, Mannheim, Germany) was performed to detect sperm DFI, according to the manufacturer’s protocol. To inhibit photobleaching of fluorescent dyes, there was the addition of VECTASHIELD Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). At least 200 cells were analyzed per slide using epifluorescent microscopy (34-36). Negative and positive controls (no TdT enzyme and treatment with DNAase, respectively) were performed in each experiment. The TUNEL negative spermatozoa fluoresced blue (spermatozoa without fragmented DNA), whereas the TUNEL-positive spermatozoa fluoresced bright green (spermatozoa with fragmented DNA). The final percentage of spermatozoa with fragmented DNA was reported for each sample.
Statistical analysis
An exploratory data analysis was carried out through measures of central position and dispersion. Comparisons between the two media (TYB and ANTIOX-PC) and fresh semen were performed using orthogonal contrasts using the mixed effects linear regression model. This model was implemented in the SAS version 9.4 program considering PROC MIXED (SAS Institute Inc., Cary, NC, USA). All data are presented as the mean ± SD. The statistically significant level was P<0.05.
Results
Clinical characteristics of subjects and fresh semen specimens
For inclusion in the research, 428 men were approached and assessed for eligibility, and among them, 307 were excluded for not meeting the inclusion criteria. At the end of the recruitment, semen samples from 121 men were subdivided according to motility in normal (n=63) and low motility (n=58), and a comparison of clinical characteristics (age, ejaculatory abstinence, semen volume and pH) of all volunteers and the sample cryopreservation times are shown in Table 1.
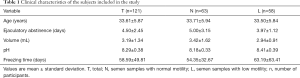
Full table
Assessment of sperm functional features
The seminal characteristics before and after freezing into the two different TYB and ANTIOX-PC cryoprotectants were analyzed, and the baseline characteristics of the semen analysis according to the WHO criteria and all parameters are shown in Tables 2 and 3.
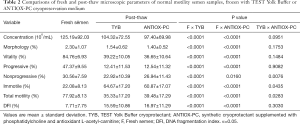
Full table
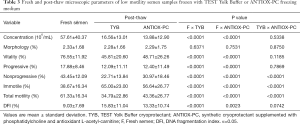
Full table
Pre-freezing sperm analysis
Regarding the main outcomes of this study, in pre-freezing samples from normal and low motility groups, sperm progressive motility rates were 47.37±9.55 and 17.88±8.46, and the DFI were 7.71±7.75 and 9.03±7.69, respectively (Tables 2,3).
Post-thawing sperm analysis
In normal motility group, all routine semen measurements after thawing were significantly lower than the pre-freeze samples not exposed to cryoprotectants and cryogenic temperatures, despite the freezing medium investigated (Table 2; P<0.001). In low motility group, the same analyzes pointed out the morphology as the only parameter unmodified after cryopreservation (P>0.05). Regarding baseline characteristics in neat semen, the sperm progressive motility in normal and low motility groups were 47.4% and 17.8%, respectively. After cryopreservation, however, we observed a more pronounced drop in progressive motility for normal than low motility group (73.8% vs. 30.3%, respectively), despite the freezing medium investigated.
Impact of cryoprotectants on sperm motility
Regarding the motility parameters investigated in both normal and low motility groups, significant differences were observed in the present study between the two cryoprotectants (Tables 2,3). Sperm from normal motility group cryopreserved in ANTIOX-PC displayed nonprogressive motility superior to TYB (26.94% vs. 22.92%; P=0.008) and quite similar rates to those observed in fresh semen (30.56%; P=0.016). On the other hand, a tendency to both decreased non-motile sperm rates (60.87% vs. 64.67%; P=0.043) and slightly increased total motility rates (39.48% vs. 35.33%; P=0.028) were observed in ANTIOX-PC compared to TYB medium, respectively. By comparing post-thawing sperm kinetics in the low motility group (asthenozoospermic samples), the non-motile sperm rates were significantly decreased whereas both nonprogressive motility and overall motility rates were significantly increased in ANTIOX-PC compared to TYB medium (P<0.0001).
Impact of cryoprotectants on sperm DNA fragmentation index
The DFI of sperm among the neat semen from normal and low motility samples, TYB, and ANTIOX-PC groups are presented in Tables 2 and 3. Sperm DNA fragmentation increased significantly after cryopreservation, despite of the baseline semen characteristics of normal and low motility groups included in this study. There was no statistically difference in the percentage of spermatozoa with fragmented DNA between TYB and ANTIOX-PC media (Tables 2,3). Regarding low motility samples, however, post-thawed sperm from ANTIOX-PC medium presented a slightly trend in DFI reduction (P=0.0742). The percentage of DNA fragmentation was 15.8%, 13.3%, and 9% for TYB, ANTIOX-PC and fresh semen, respectively (Table 3) in these samples. Although the DFI differences between the two freezing media evaluated were not significant, the DFI increased by about 75.8%, and 47.8% in spermatozoa recovered from TYB and ANTIOX-PC, respectively. Representative TUNEL images of post-thaw human spermatozoa from low motility samples cryopreserved in TYB and ANTIOX-PC media are shown in Figure 1.
Discussion
In this study we examined the progressive motility recovery rates and DNA integrity index of human spermatozoa from men with normospermia or asthenozoospermia, after freeze-thaw cycles using a synthetic soy-PC and L-acetyl-carnitine-based cryoprotectant (ANTIOX-PC) or the conventional egg yolk freezing medium (TYB). The main findings of this study were no statistically differences in the percentage of normal spermatozoa with progressive motility and DNA integrity recovered after thawing of cryopreserved sperm in ANTIOX-PC compared to TYB medium.
Sperm quality parameters such as sperm motility can affect the cryosurvival rate of post-thawing spermatozoa (37). Considering the effects of pre-freezing conditions on the progressive motility recovery rate of human sperm cryopreserved, our results showed a marked decrease of 73.8% of progressive motility in samples from the normal motility group compared to 30.3% of the low motility group, regardless of the freezing medium used. As previously reported, the progressive motility rates of the cryopreserved sperm were not correlated with pre-freezing semen parameters, such as sperm concentration, progressive motility, and morphology (38).
Cryopreservation of human spermatozoa has been associated with increased susceptibility to DNA fragmentation (39). In fact, our results demonstrated that the percentage of spermatozoa with fragmented DNA after freezing-thawing were doubled in normal motility semen samples for both TYB and ANTIOX-PC groups. In general, the percentage of sperm with fragmented DNA dramatically increased in low motility group after cryopreservation in our study; however, a trend to lower DFI was observed in post-thaw sperm from ANTIOX-PC. Although no statistical difference was detected between the cryoprotectant groups, the ANTIOX-PC medium provided 28% more sperm with non-fragmented DNA than TYB, evidencing the clinical relevance of this result and the advantage of the ANTIOX-PC medium in better preserving the chromatin integrity of spermatozoa from men with low motility semen.
Furthermore, a significantly increase in overall motility was observed in ANTIOX-PC medium. Remarkably, an increase in nonprogressive motility in parallel with a lower percentage of immotile spermatozoa recovered from ANTIOX-PC contributed to an increased total sperm motility when compared to TYB medium in both normal and low motility groups. A strong correlation between the percentage of immotile spermatozoa and mitochondrial defects after thawing has been reported (40). Therefore, recovery of an optimal number of functionally intact spermatozoa from thawed samples has been the main objective of semen cryopreservation technology. Although the percentage of spermatozoa with progressive motility in the ANTIOX-PC group was lower compared to the neat semen and did not differ from the TYB medium, the total motility was significantly increased after the PC and L-acetyl-carnitine treatment of the sperm in both normal and low motility semen samples.
Sperm functional parameters as the DNA integrity and motility have been demonstrated to be more linked to sperm fertility (41-43). Indeed, fertilization of oocytes by sperm with damaged DNA may lead to abnormal embryo development (44), increased risk of abortion (45), mutations and risk of cancer in the offspring (46). Therefore, improvements in these sperm functional parameters after cryopreservation are clinically relevant to ART, especially in men with poor semen quality (47). Notably, our results showed that the total motility of post-thawing spermatozoa from men with low motility decreased by 29.3% in ANTIOX-PC medium compared to initial total motility of the fresh semen. On the other hand, the overall motility was reduced by 43.2% in sperm cryopreserved in TYB compared to net semen. This finding indicates that the L-acetyl-carnitine added to ANTIOX-PC may be improved total motility recovery in samples with low degree of motility. Membrane lipid peroxidation and disrupted energy metabolism are major events leading to sperm cell death after cryopreservation. These cooperative pathways share as one common aspect the triggering of oxidative stress by free radical formation. L-carnitine is an antioxidant agent used in the treatment of men with low seminal quality removing the toxic excess of intracellular acetyl-CoA and protecting the spermatozoa from oxidative damage (27-29). Moreover, carnitines play a key role in energy metabolism, transferring free fatty acids from the cytosol to mitochondria, facilitating their oxidation and generation of adenosine triphosphate (27,30). In cardiac cells, L-carnitine is essential for mitochondria function, to attenuate the membrane permeability transition, and to maintain the ultrastructure and membrane stabilization, in the presence of high fatty acid β-oxidation (37).
In sperm samples characterized by asthenozoospermia, L-acetyl carnitine/L-carnitine ratio and acetylation rate are markedly reduced (47,48). In addition, a prospective clinical trial reported that sperm motility increased 99.6% in asthenozoospermic men after oral LC treatment (49). L-acetyl-carnitine plays a crucial role on sperm metabolism, including sperm motility. By interaction with CoA, carnitine is involved in the intermediary metabolism by modulating free CoA pools in the sperm with detoxification and anabolic properties, besides its antioxidant and antiapoptotic roles (30). Additionally, Lα-phosphatidylcholine by its amphipathic and surfactant nature promotes the formation of a protective film (micellar composition) to the spermatozoa, which may avoid mechanical damages in the sperm membrane, contributing to the increase of sperm cryotolerance and its survival after the freeze-thawing procedure (24).
The egg yolk cryopreservation media are still widely used in ART due to their effectiveness in protecting the spermatozoa during cryopreservation (26), as well as important protective function of the plasma membrane, preventing thermal shock and improving sperm motility (18). However, its use has been revised due to the biosafety aspects, with potential risk of transmitting infectious agents and variations in its composition (11,19,22,50,51). The advantages of a PC based cryoprotectant obtained from soybean are related to its defined chemical composition, allowing the identification of all the components added to the medium. Its synthetic composition also makes it possible to obtain standardized procedures, accurate information about its mechanisms of action in spermatozoa, longer shelf life due to storage free of degradation, and reduces the potential risk of microbiological contamination due to the absence of animal-based additives.
In our study, the efficacy of soy-PC and L-acetyl-carnitine supplements in human semen cryopreservation could be tested and demonstrated for the first time in a study with a sample size of 121 men including different degrees of sperm motility. Previous studies investigating the efficacy of egg yolk-free cryopreservation media were predominantly conducted with normozoospermic semen samples and presented a series of 20 to 28 subjects (19-21,24,52).
The study limitations are related to the phospholipid micelles formed by the ANTIOX-PC medium, which made it difficult to analyze the immotile sperm due its overlapping with micelles above 50 µm. In addition, micelles revealed the cryoprotective medium being analyzed, compromising the blinding of the study. As well, the average percentage of sperm with normal morphology was not normal in any of the groups, even before cryopreservation. Furthermore, our study is primarily laboratory based and more studies need to be undertaken to investigate the post-thaw motility of the human spermatozoa cryopreserved in ANTIOX-PC medium at different thawing temperatures and also after prolonged post-thaw intervals as well as in terms of pregnancy in IUI cycles or fertilization rate in IVF/ICSI cycles.
In conclusion, we show here that soy-PC and L-acetyl-carnitine can successfully replace egg yolk as supplements for cryopreservation medium, without adverse effects on post-thaw sperm motility, morphology, vitality, and sperm chromatin fragmentation index. The investigation of the impact of a synthetic cryoprotectant formulation on the preservation of the semen quality of men with low sperm motility is novel and may potentially contribute to the improvement of the cryopreservation of low-quality seminal samples in ART, semen banks and male fertility preservation.
Acknowledgments
The authors would like to thank the study participants and Marilda Hatsumi Yamada Dantas, Sônia Aparecida Scandiussi Andrade, Elizabete Rosa Milani, and Suleimy Mazin support.
Funding: This work was supported by São Paulo Research Foundation (FAPESP) (grant number 2014/13477-2) and National Council for Scientific and Technological Development - CNPq for financial support.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1004
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1004
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-1004
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1004). AAV and RMR have a patent BR 13 2018 016456-1 pending, and a patent BR 1020130192139B1 granted to University of São Paulo. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a prospective experimental study that was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Local Research Ethics Committee and also by the National Commission for Ethics in Research (Protocols: CAAE 44677215.0.0000.5440 and CAAE 35850214.4.0000.5440) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hezavehei M, Sharafi M, Kouchesfahani HM, et al. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod Biomed Online 2018;37:327-39. [Crossref] [PubMed]
- Anger JT, Gilbert BR, Goldstein M. Cryopreservation of sperm: indications, methods and results. J Urol 2003;170:1079-84. [Crossref] [PubMed]
- Stensvold E, Magelssen H, Oskam IC. Fertility-preserving measures for boys and young men with cancer. Tidsskr Nor Laegeforen 2011;131:1433-5. [Crossref] [PubMed]
- Varghese AC NP, Mahfouz R, Athayde KS, et al. Human sperm cryopreservation. Andrology Laboratory manual. New Delhi: Brothers Medical Publishers Ltd., 2010.
- Benson JD, Woods EJ, Walters EM, et al. The cryobiology of spermatozoa. Theriogenology 2012;78:1682-99. [Crossref] [PubMed]
- Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev 2001;59:451-8. [Crossref] [PubMed]
- O'Connell M, McClure N, Lewis SE. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 2002;17:704-9. [Crossref] [PubMed]
- Srisombut C, Morshedi M, Lin MH, et al. Comparison of various methods of processing human cryopreserved-thawed semen samples. Hum Reprod 1998;13:2151-7. [Crossref] [PubMed]
- Borges E, Rossi LM, Locambo de Freitas CV, et al. Fertilization and pregnancy outcome after intracytoplasmic injection with fresh or cryopreserved ejaculated spermatozoa. Fertil Steril 2007;87:316-20. [Crossref] [PubMed]
- Watson PF. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev 1995;7:871-91. [Crossref] [PubMed]
- Ricker JV, Linfor JJ, Delfino WJ, et al. Equine sperm membrane phase behavior: the effects of lipid-based cryoprotectants. Biol Reprod 2006;74:359-65. [Crossref] [PubMed]
- Thomson LK, Fleming SD, Aitken RJ, et al. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 2009;24:2061-70. [Crossref] [PubMed]
- Bollwein H, Fuchs I, Koess C. Interrelationship between plasma membrane integrity, mitochondrial membrane potential and DNA fragmentation in cryopreserved bovine spermatozoa. Reprod Domest Anim 2008;43:189-95. [Crossref] [PubMed]
- Brugnon F, Ouchchane L, Pons-Rejraji H, et al. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum Reprod 2013;28:2045-57. [Crossref] [PubMed]
- Calamera JC, Buffone MG, Doncel GF, et al. Effect of thawing temperature on the motility recovery of cryopreserved human spermatozoa. Fertil Steril 2010;93:789-94. [Crossref] [PubMed]
- Isachenko E, Isachenko V, Katkov II, et al. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum Reprod 2004;19:932-9. [Crossref] [PubMed]
- Petyim S, Neungton C, Thanaboonyawat I, et al. Sperm preparation before freezing improves sperm motility and reduces apoptosis in post-freezing-thawing sperm compared with post-thawing sperm preparation. J Assist Reprod Genet 2014;31:1673-80. [Crossref] [PubMed]
- Bergeron A, Manjunath P. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev 2006;73:1338-44. [Crossref] [PubMed]
- Jeyendran RS, Acosta VC, Land S, et al. Cryopreservation of human sperm in a lecithin-supplemented freezing medium. Fertil Steril 2008;90:1263-5. [Crossref] [PubMed]
- Mutalik S, Salian SR, Avadhani K, et al. Liposome encapsulated soy lecithin and cholesterol can efficiently replace chicken egg yolk in human semen cryopreservation medium. Syst Biol Reprod Med 2014;60:183-8. [Crossref] [PubMed]
- Reed ML, Ezeh PC, Hamic A, et al. Soy lecithin replaces egg yolk for cryopreservation of human sperm without adversely affecting postthaw motility, morphology, sperm DNA integrity, or sperm binding to hyaluronate. Fertil Steril 2009;92:1787-90. [Crossref] [PubMed]
- Bousseau S, Brillard JP, Marguant-Le Guienne B, et al. Comparison of bacteriological qualities of various egg yolk sources and the in vitro and in vivo fertilizing potential of bovine semen frozen in egg yolk or lecithin based diluents. Theriogenology 1998;50:699-706. [Crossref] [PubMed]
- Szuhaj B. Phospholipids: properties and occurrence. Encyclopedia of Food Sciences and Nutrition 2003:4514-9.
- Vireque AA, Tata A, Silva OF, et al. Effects of n-6 and n-3 polyunsaturated acid-rich soybean phosphatidylcholine on membrane lipid profile and cryotolerance of human sperm. Fertil Steril 2016;106:273-83.e6. [Crossref] [PubMed]
- BR1020130192139. Composição sintética para criopreservação de sêmen; seu processo de preparação e uso, 2013. Brasil. Reis RM, Vireque AA, Dantas MHY, Silva OFLLO. Universidade de São Paulo.
- Del Valle I, Gómez-Durán A, Holt WV, et al. Soy lecithin interferes with mitochondrial function in frozen-thawed ram spermatozoa. J Androl 2012;33:717-25. [Crossref] [PubMed]
- Balercia G, Regoli F, Armeni T, et al. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril 2005;84:662-71. [Crossref] [PubMed]
- Lenzi A, Sgrò P, Salacone P, et al. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril 2004;81:1578-84. [Crossref] [PubMed]
- Sigman M, Glass S, Campagnone J, et al. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2006;85:1409-14. [Crossref] [PubMed]
- Jeulin C, Lewin LM. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum Reprod Update 1996;2:87-102. [Crossref] [PubMed]
- BR13 2018 016456-1. Composição micelar antioxidante para criopreservação e processamento de sêmen e seu uso, 2019. Brasil. Reis RM, Vireque AA. Universidade de São Paulo.
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th edn 2010.
- Kruger TF, Acosta AA, Simmons KF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988;49:112-7. [Crossref] [PubMed]
- Berteli TS, Da Broi MG, Martins WP, et al. Magnetic-activated cell sorting before density gradient centrifugation improves recovery of high-quality spermatozoa. Andrology 2017;5:776-82. [Crossref] [PubMed]
- Delbes G, Herrero MB, Troeung ET, et al. The use of complimentary assays to evaluate the enrichment of human sperm quality in asthenoteratozoospermic and teratozoospermic samples processed with Annexin-V magnetic activated cell sorting. Andrology 2013;1:698-706. [Crossref] [PubMed]
- Fortunato A, Leo R, Casale S, et al. Sperm DNA Fragmentation Assays Correlate with Sperm Abnormal Morphology and the Pregnancy Outcome | OMICS International. Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biology 2013;1:101. [Crossref]
- Oyanagi E, Yano H, Uchida M, et al. Protective action of L-carnitine on cardiac mitochondrial function and structure against fatty acid stress. Biochem Biophys Res Commun 2011;412:61-7. [Crossref] [PubMed]
- Lee CY, Lee CT, Wu CH, et al. Kruger strict morphology and post-thaw progressive motility in cryopreserved human spermatozoa. Andrologia 2012;44 Suppl 1:81-6. [Crossref] [PubMed]
- Dalzell LH, McVicar CM, McClure N, et al. Effects of short and long incubations on DNA fragmentation of testicular sperm. Fertil Steril 2004;82:1443-5. [Crossref] [PubMed]
- Ozkavukcu S, Erdemli E, Isik A, et al. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet 2008;25:403-11. [Crossref] [PubMed]
- Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 2001;76:892-900. [Crossref] [PubMed]
- Elbashir S, Magdi Y, Rashed A, et al. Relationship between sperm progressive motility and DNA integrity in fertile and infertile men. Middle East Fertil Soc J 2018.195-8. [Crossref]
- Gómez-Torres MJ, Medrano L, Romero A, et al. Effectiveness of human spermatozoa biomarkers as indicators of structural damage during cryopreservation. Cryobiology 2017;78:90-4. [Crossref] [PubMed]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289-95. [Crossref] [PubMed]
- Twigg J, Fulton N, Gomez E, et al. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 1998;13:1429-36. [Crossref] [PubMed]
- Aitken RJ, Smith TB, Jobling MS, et al. Oxidative stress and male reproductive health. Asian J Androl 2014;16:31-8. [Crossref] [PubMed]
- Kohengkul S, Tanphaichitr V, Muangmun V, et al. Levels of L-carnitine and L-O-acetylcarnitine in normal and infertile human semen: a lower level of L-O-acetycarnitine in infertile semen. Fertil Steril 1977;28:1333-6. [Crossref] [PubMed]
- Bartellini M, Canale D, Izzo PL, et al. L-carnitine and acetylcarnitine in human sperm with normal and reduced motility. Acta Eur Fertil 1987;18:29-31. [PubMed]
- Vitali G, Parente R, Melotti C. Carnitine supplementation in human idiopathic asthenospermia: clinical results. Drugs Exp Clin Res 1995;21:157-9. [PubMed]
- Chelucci S, Pasciu V, Succu S, et al. Soybean lecithin-based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology 2015;83:1064-74. [Crossref] [PubMed]
- Nishijima K, Kitajima S, Koshimoto C, et al. Motility and fertility of rabbit sperm cryopreserved using soybean lecithin as an alternative to egg yolk. Theriogenology 2015;84:1172-5. [Crossref] [PubMed]
- Tiwari A, Tekcan M, Sati L, et al. A new media without animal component for sperm cryopreservation: motility and various attributes affecting paternal contribution of sperm. J Assist Reprod Genet 2017;34:647-57. [Crossref] [PubMed]


