
18F-PSMA-1007 PET/CT uptake in multiple angiolipomas caused by PSMA expression in capillaries: a case report
Introduction
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that was originally cloned in the membrane of prostate gland epithelial cells. PSMA expression increases 100–1,000 times in advanced prostate cancer cells, hence the name, PSMA (1). The strong expression of PSMA in prostate cancer is associated with a high serum prostate-specific antigen (PSA) level, high Gleason score, advanced tumor progression, negative prognosis, and has been substantiated as an independent predictor of tumor recurrence (2), therefore supporting PSMA radioligand-based imaging and therapy for the management of prostate cancer (3). Interestingly, recent studies have reported that PSMA is expressed in other genitourinary malignancies, particularly renal cell carcinoma (4). Enhanced uptake on PSMA-based positron emission tomography/computed tomography (PET/CT) scans have also been ascribed to some benign lesions (e.g., in ganglia or healing rib fractures) (5). There are many reasons for the high expression of PSMA in these lesions. Angiolipoma is a special type of lipoma. It is a rare benign neoplasm which is generally found in subcutaneous tissues. Surgical removal is possible if necessary, and the final diagnosis is dependent on histopathological evaluation (6,7). Angiolipoma is a special type of lipoma that is formed by mixing mature adipose tissue with hyperactive vascular tissue. In the literature, there are only two reports of angiolipomas as determined by 68Ga-PSMA and fluorodeoxyglucose (18F-FDG) PET/CT (8,9), although the possible reasons for enhanced uptake of PSMA in these angiolipomas are not explained. Herein, we report a rare case of an elderly male patient with multiple angiolipomas, which were detected by 18F-PSMA1007 PET/CT. Most importantly, we performed a histopathological evaluation and immunohistochemical staining of the angiolipoma lesions and found a possible cause of its high uptake of PSMA. We present the following case in accordance with the CARE reporting checklist (10) (available at http://dx.doi.org/10.21037/tau-20-1099).
Case presentation
A 77-year-old man presented with frequent urination and dysuria which had persisted for 8 months. Physical examination revealed multiple painless nodules distributed subcutaneously in the lower back, upper back, shoulders, and upper arms. There was no palpable lymphadenopathy in the inguinal region. Past medical and family history were unremarkable. The serum PSA level was 27.7 ng/mL. Magnetic resonance imaging (MRI) was followed by a biopsy, which confirmed prostate cancer (Gleason score 8). The patient then underwent a 18F-PSMA-1007 PET/CT scan for staging. The maximum intensity projection image (MIP) showed intense focal PSMA uptake in the whole enlarged prostate gland, indicating locally advanced prostate cancer (Figure 1). Histopathological examination also revealed prostate cancer, as significant PSMA expression in prostate cancer tissue was observed (Figure 2). Additionally, the PET/CT scan demonstrated multiple PSMA-avid subcutaneous fatty density nodules (Figure 1E,F,G), which had spread over the areas mentioned above. The highest level of uptake occurred in the nodules of the right lower back, with a SUVmax value of 8.2. The patient underwent radical prostatectomy 3 days after the scan, and 3 lesions (bilateral back and left forearm) were excised.
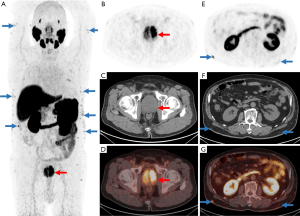
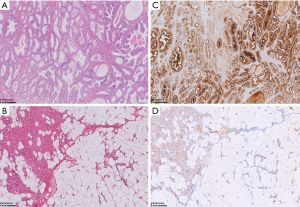
After incision, the nodules had an obvious envelope and were lobulated. The cut surface was yellow, and the edges were dark red due to blood vessel components. Microscopically, in addition to mature lobulated adipose in the tissues, there were still proliferating capillaries that grew from the capsule along the septal connective tissue to the center. Endothelial cells proliferated and the lumen was narrow. Some could only accommodate 1 to 2 red blood cells or were completely occluded (Figure 2B). The collagen fibers in the interstitium were homogeneous, lightly stained red with eosin, and had no signs of inflammation or malignancy such as increased cell proliferation, nuclear polymorphisms, or mitotic activity. These features were consistent with angiolipoma (11). PSMA immunoreactivity results are shown in Figure 2, which demonstrated strong positive cytoplasmic PSMA staining in lesional prostate cancer cells in the prostate carcinoma and mild-to-moderate positive cytoplasmic capillary PSMA staining in the angiolipoma lesion. Mature adipocytes did not express PSMA (Figure 2D). Based on the 18F-PSMA PET/CT imaging, operative, and histological findings, the final diagnosis was prostate cancer with multiple subcutaneous angiolipomas. PSMA-based PET/CT uptake in angiolipomas was caused by PSMA expression in capillaries or the vasculature. The timeline of the case is depicted in Figure 3.
Study protocols accorded with recommendations of the Commission of Medical Research Involving Human Subjects at Region of Xiangya Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
PSMA is a type II transmembrane glycoprotein, which consists of 19 intracellular amino acids, 24 transmembrane amino acids, and 707 extracellular amino acids. It is physiologically expressed in epithelial cells around the top of the prostate duct, and in some other tissues in the cytoplasm (e.g., renal cortex, duodenum, ileum, salivary glands, lacrimal glands, and coeliac ganglia). Functionally, it has both folate hydrolase activity and N-acetylated α-linked acidic dipeptidase (NAALADase) activity (1,4). In dysplastic or cancerous tissues, PSMA can metastasize from the apical membrane to the lumen surface of the catheter, and the tumor tissues that transform to androgen-independent prostate cancer have a higher expression of PSMA. In addition, PSMA is also expressed in other tumor tissues or neovascular tissues other than prostate cancer, including lung cancers, colonic adenocarcinomas, clear cell renal carcinomas, transitional cell carcinomas, schwannomas, malignant melanomas, and osteosarcomas (12). The histopathological expression of PSMA in these malignancies is usually confined to the neovasculature rather than to the tumor cells. PSMA uptake intensity is also weaker, and the patterns of distribution are very different from those of prostate cancer. Possible pathophysiological mechanisms include the increase in local folic acid concentration in the tissue from the folate hydrolase activity of PSMA (1,13). Folic acid increases the level of nitric oxide by promoting the synthesis of endothelial nitric oxide synthase, which ultimately facilitates angiogenesis (14). Tumor-associated neovascularization is further related to high aggressiveness, high metastasis ability, and unfavorable prognosis (15). In general, high neovascular PSMA expression is more common in malignant tumors than in moderate or benign tumors (4).
Angiolipoma is a special type of lipoma that is formed by a mixture of mature adipocytes and abnormally proliferating angiomas. It is generally seen in young men, and is a benign tumor that grows very slowly (16). The presence of fibrinous hyaline thrombi in the vascular cavity is an important feature for the diagnosis of angiolipoma (11). Angiolipoma in an older patient is a very rare phenomenon, and only 2 cases, as diagnosed by PET/CT, have been previously reported. In one case, the patient had a focal angiolipoma with increased 18F-FDG PET uptake in the left lower abdominal wall. The authors thought this might have been due to hypervascularity, blood stasis, and congestion in the vascular structure, which accelerates inflammatory processes and leads to high 18F-FDG uptake (9). Another case report found high uptake of 68Ga-PSMA in subcutaneous angiolipoma, but did not provide further in-depth analysis of the potential causes (8). Our case report demonstrated that 18F-PSMA-1007 PET uptake in angiolipoma is associated with PSMA expression levels in the capillaries or vasculature. The cause of high uptake of PSMA in benign lesions has not been thoroughly investigated in previous studies. Hofvander et al. found low-level protein kinase D2 (PRKD2) gene mutations in angiolipomas (17). PRKD2 is a main component of a regulatory loop that tightly modulates β1 integrins and PSMA. Active PSMA facilitates integrin signal transduction, endothelial nitric oxide synthase regeneration, and p21-activated kinase (PAK) activation, leading to increased invasion and adhesion of endothelial cells, angiogenesis, and distorted capillary networks within the angiolipomas (18,19).
PSMA, also known as glutamate carboxypeptidase II, has been shown to not only be highly expressed in prostate cancer, but also in various non-prostatic tissues, including the brain and other benign processes. Understanding the physiological distribution of PSMA and other causes for uptake is therefore necessary to minimize false-positive imaging findings. Recently, 18F-labeled PSMA has been shown to be a promising labelling strategy and potential clinical alternative for 68Ga-labeled counterparts. There are some major principle advantages of radiofluorinated tracers over 68Ga-labeled PSMA ligands, such as a longer half-life (110 vs. 68 min), centralized production and distribution leading to cost savings, the possibility of large-batch production (cyclotron-produced 18F vs. generator-produced 68Ga), and the lower positron energy of 18F compared to 68Ga, potentially improving spatial resolution and reducing blurring effects. However, studies on biochemical recurrence after radical prostatectomy have shown that the number of benign lesions with increased PSMA-ligand uptake and the number of recurrent lesions were both considerably higher for 18F-PSMA PET/CT than for 68Ga-PSMA PET/CT (20,21). This needs to be verified by further studies with larger sample sizes, and also indicates that we need more sophisticated reader training to avoid pitfalls and reduce false–positive rates as much as possible.
Many questions remain to be answered, among which include the method for confirming the final pathological results of lesions in which the uptake of two isotope-labeled PSMA ligands are inconsistent and cannot be biopsied. This case report had some limitations. Multiple subcutaneous angiolipomas are a rare type of benign tumor, and so the high uptake or expression of capillary PSMA in this case of angiolipoma can only provide an interpretation in the context of benign tumors with a high expression of PSMA. More benign lesions with a high uptake of PSMA and possible causes need to be further investigated in order to minimize false-positive imaging findings.
Conclusions
In summary, apart from the known high uptake of PSMA in prostate carcinoma, our study demonstrated that 18F-PSMA-1007 PET/CT uptake in multiple angiolipomas can be caused by PSMA expression in capillaries. Further knowledge regarding the causes of PSMA uptake is essential for minimizing false-positive imaging isotope-labeled PSMA PET findings.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (No. 91859207, 81771873, and 81471689).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1099
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1099). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Study protocols accorded with recommendations of the Commission of Medical Research Involving Human Subjects at Region of Xiangya Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997;3:81-5. [PubMed]
- Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 2003;9:6357-62. [PubMed]
- Hofman MS, Violet J, Hicks RJ, et al. [Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018;19:825-33. [Crossref] [PubMed]
- Siva S, Udovicich C, Tran B, et al. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol 2020;17:107-18. [Crossref] [PubMed]
- Hofman MS, Hicks RJ, Maurer T, et al. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018;38:200-17. [Crossref] [PubMed]
- Lin JJ, Lin F. Two entities in angiolipoma. A study of 459 cases of lipoma with review of literature on infiltrating angiolipoma. Cancer 1974;34:720-7. [Crossref] [PubMed]
- Lou XH, Chen WG, Ning LG, et al. Multiple gastric angiolipomas: A case report. World J Clin Cases 2019;7:778-84. [Crossref] [PubMed]
- Dekker I, van der Leest M, van Rijk MC, et al. 68Ga-PSMA Uptake in Angiolipoma. Clin Nucl Med 2018;43:757-8. [Crossref] [PubMed]
- Hsu CH, Yang CM, Cheng CJ. Angiolipoma detected by F-18 FDG positron emission tomography. Clin Nucl Med 2003;28:604-5. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Charifa A, Badri T. Lipomas, Pathology. StatPearls. Treasure Island (FL) 2019.
- Heitkötter B, Trautmann M, Grunewald I, et al. Expression of PSMA in tumor neovasculature of high grade sarcomas including synovial sarcoma, rhabdomyosarcoma, undifferentiated sarcoma and MPNST. Oncotarget 2017;8:4268-76. [Crossref] [PubMed]
- Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur Urol 2019;76:469-78. [Crossref] [PubMed]
- Ghosh A, Heston WDW. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 2004;91:528-39. [Crossref] [PubMed]
- Zeng C, Ke ZF, Yang Z, et al. Prostate-specific membrane antigen: a new potential prognostic marker of osteosarcoma. Med Oncol 2012;29:2234-9. [Crossref] [PubMed]
- Howard WR, Helwig EB. Angiolipoma. Arch Dermatol 1960;82:924-31. [Crossref] [PubMed]
- Hofvander J, Arbajian E, Stenkula KG, et al. Frequent low-level mutations of protein kinase D2 in angiolipoma. J Pathol 2017;241:578-82. [Crossref] [PubMed]
- Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 2006;26:5310-24. [Crossref] [PubMed]
- Onodera Y, Nam JM, Hashimoto A, et al. Rab5c promotes AMAP1-PRKD2 complex formation to enhance β1 integrin recycling in EGF-induced cancer invasion. J Cell Biol 2012;197:983-96. [Crossref] [PubMed]
- Rauscher I, Kronke M, Konig M, et al. Matched-pair comparison of (68)Ga-PSMA-11 and (18)F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med 2020;61:51-7. [Crossref] [PubMed]
- Giesel FL, Knorr K, Spohn F, et al. Detection Efficacy of F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J Nucl Med 2019;60:362-8. [Crossref] [PubMed]



