
Assessment of prognosis by established prognosis scores and physicians’ judgement in mRCC patients: an analysis of the STAR-TOR registry
Introduction
Renal cell carcinoma (RCC) is the 10th most often cancer in women and 7th most often in men and is estimated to be the 11th most leading cause of death in the united states in 2015 (1). In the majority of cases RCC can be cured with (partial) nephrectomy. However, locally advanced and metastatic RCC (a/mRCC) remains an incurable disease. In recent years significant progress has been made and several compounds with different methods of action have been approved to either improve on progression-free survival (PFS) and/or overall survival (OS) (2-12).
Several prognostic scoring systems are currently used to guide physicians in performing treatment decisions. The Memorial Sloan-Kettering Cancer Center-Score (MSKCC), first described for prognostication of OS in patients with a/mRCC in the era in which treatment was primarily done with interferon-α, uses five risk-factors (low Karnofsky performance status (<80%), high serum lactate dehydrogenase (>1.5 times upper limit of normal), low hemoglobin (< lower limit of normal), high corrected serum calcium (>10 mg/dL) and time from diagnosis of RCC to start of systemic therapy <1 year) (13). In the scoring system, no risk factor represents good prognosis while 1–2 and 3 risk factors represent intermediate and poor prognosis, respectively. The MSKCC-score has been shown to be also valid in the era of targeted therapy in 2011 (14). Another widely used score, the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC)-score proposed by Heng et al., uses four of the MSKCC-criteria (hemoglobin less < lower limit of normal, corrected calcium >10 mg/dL, Karnofsky performance status <80%, and time from diagnosis to treatment <1 year. In addition, neutrophils > upper limit of normal (UNL) and platelets > UNL were added to a model that showed to be prognostic for OS (15). Here, as for the MSKCC-score, no risk factor represents good prognosis and 1–2 and 3 risk factors intermediate and poor prognosis, respectively.
Temsirolimus (TEMS), an i.v. mechanistic Target of Rapamycin (mTOR)-inhibitor, is approved for the first-line treatment of patients with a/mRCC who have at least 3 of 5 risk factors according to the MSKCC-score or 3 of 6 risk factors according to the IMDC-score representing poor prognosis (13,15). A pivotal study demonstrated significantly increased OS with TEMS in poor risk a/mRCC compared to the former standard interferon-α (10.9 vs. 7.3 months) (5). Within this trial, another prognostic score was used (Hudes-Score). Here, the five MSKCC-criteria plus 2 sites of organ metastasis are the risk factors used. According to these Hudes-criteria 3 risk-factors represent poor prognosis and <3 factors non-poor prognosis, respectively.
Especially the MSKCC- and IMDC-scores are currently most widely used for prognostication of OS and used in several international guidelines to steer therapy decisions. The guidelines of the European Society of Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) currently recommend using TEMS as single 1st line therapy in patients with clear cell RCC and poor prognosis according to the IMDC/MSKCC-criteria in which an immunotherapy-containing therapy is deemed not feasible (16,17). The National Comprehensive Cancer Network (NCCN)-guidelines equally currently give a category 1 recommendation to use TEMS in poor risk patients with predominantly clear cell histology based on the MSKCC-criteria (18). Because of these guideline recommendations and despite of the plethora of available compounds for the treatment of RCC, TEMS remains a backbone of therapy of RCC.
Next to the prognostic scores which can be calculated using objectively measurable facts, the physicians treating the patients will always use softer criteria to assess the status and the prognosis of the patient. Amongst others he will consider facts like, visual appearance, pain, appetite or motion ability of the respective patient or information he gets when asking the patient and relatives or, last but not least, his own gut feeling.
To the best of our knowledge the physician’s assessment of prognosis has not been compared with objective scores in patients with a/mRCC, yet.
The StarTOR-study, a German multicenter registry for patients with a/mRCC (NCT00700258) was established in 2008 to evaluate the safety and efficacy of TEMS in a routine clinical setting.
We assessed the prognostic value of the MSKCC-, IMDC- and Hudes-score in the TEMS treated cohort of the StarTOR-registry and compared the results with the physician’s prognosis. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-938).
Methods
Patients
From February 2008 until May 2015, 547 patients with a/mRCC were prospectively included into the StarTOR registry and treated with TEMS in 114 German centers. The study protocol was approved by the ethics committee of the Medical Board of Westfalia-Lippe and the Westfalian Wilhelms-University in Muenster (Approval number: 2007-484-f-S) and all patients had given written informed consent prior to any study-specific actions. Before first infusion of TEMS the respective attending physicians were asked to give their own prognosis of the patients. The study conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013.
TEMSR 25 mg weekly was applied until disease progression, intolerable toxicity or treatment discontinuation by the physician or the patient for other reasons.
Because of the non-interventional design of the study, the physician was responsible for dose reductions, treatment breaks and for the intervals of cross-sectional imaging, which did not exceed three months in almost all cases. The radiographic response was assessed by the attending physicians according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (19). After baseline workup of clinical and laboratory information, reevaluations were mostly done in weekly but in no more than four weekly intervals.
These data were analyzed to compare established prognostic scores with the risk assessment attributed to patients by the attending physicians and match them with survival outcomes. The purpose of this analysis was to compare different prognostic systems in the treatment of a/mRCC with each other and with the individual prognosis the physician makes for each patient by his medical expertise.
Statistical methods
The compilation of collected data was carried out by the independent clinical research organization Winicker Norimed (Nuremberg, Germany). For descriptive statistics, we report medians with 95% confidence intervals (95% CI) or interquartile range (IQR) for continuous variables and populations and frequencies for categorical variables. We determined the significance of the differences between categorical and continuous variables using the χ2-test, Fisher’s exact-test or Mann-Whitney U-test. For analysis of survival outcomes, we applied Kaplan-Meier-estimates and for univariate and multivariate analysis Cox-regression-models. PFS was defined as the time from start of TEMS-treatment till radiographic progression, unequivocal clinical progression or death under TEMS-treatment. OS was defined as the time from start of TEMS-treatment until death from any cause. All reported p-values are two-sided, and we assumed statistical significance when P was ≤0.05. We used SPSS-Statistics V.23 (IBM Inc., Armonk, NY, USA) for statistical assessment.
Results
Patient characteristics
The study population of 547 patients was, as expected for this tumor entity: 68.3% male, 77.1% with clear cell histology, 46.9% had a Karnofsky performance score <80%. Table 1 shows the patient- and tumor-specific details. Treatment characteristics are given in Table 2. Pretreatment had been done in 299 (54.7%) patients, most of them with Sunitinib and 29% of patients received TEMS in third or later lines. At a median follow-up of 4.7 months (IQR: 2.3–6.7 months), the clinical benefit rate (complete or partial remission or stable disease) was 64.7%. The median duration of treatment with TEMS was 3.3 months (IQR: 1.5–7.9 months) and the median dose intensity was 89,9% (IQR: 77.2–100%). The median PFS for the whole study population was 4.4 months (95% CI: 3.7–5.1 months) and the median OS was 10.6 months (95% CI: 8.9–12.3 months). At the time of analysis 77.5% of patients had discontinued TEMS-treatment.
Table 1
| Variable | No (%) |
|---|---|
| Total number of patients | 547 |
| Sex (n=543) | |
| Male | 371 (68.3) |
| Female | 172 (31.7) |
| Histologic subtype (n=530) | |
| Clear cell | 422 (77.1) |
| Papillary | 65 (11.9) |
| Chromophobe | 12 (2.2) |
| Collecting duct | 3 (0.5) |
| Uncertain but non-clear cell/others | 28 (5.1) |
| Number of organ systems with metastases | |
| 1 | 141 (25.8) |
| 2 | 185 (33.8) |
| 3 | 221 (40.4) |
| Karnofsky performance status prior to start of therapy (n=535) | |
| <80% | 251 (46.9) |
| 80% | 284 (53.1) |
| Localization of metastases | |
| Lung | 370 (67.6) |
| Lymph nodes | 242 (44.2) |
| Bone | 197 (36.0) |
| Liver | 127 (23.2) |
| Adrenal gland | 72 (13.2) |
| Kidney (contralateral) | 41 (7.5) |
| Central nervous system | 33 (6.0) |
| Other | 135 (24.7) |
| LDH in serum (n=412) | |
| ≤300 U/L | 304 (73.8) |
| >300 U/L | 108 (26.2) |
No, number; LDH, lactate dehydrogenase.
Table 2
| Variable | No (%) |
|---|---|
| Primary tumor resected (n=546) | |
| Yes | 466 (85.3) |
| No | 80 (14.7) |
| Nephrectomy (n=546) | |
| Total | 439 (80.4) |
| Partial | 27 (4.9) |
| No | 80 (14.7) |
| Previous radiation therapy (n=532) | |
| No | 380 (71.4) |
| Yes | 152 (28.6) |
| Line of Temsirolimus therapy (n=544) | |
| First line | 242 (44.5) |
| Second line | 144 (26.5) |
| Third or later line | 158 (29.0) |
| Systemic pretreatment (n=299) | |
| Cytokines | 75 (25.1) |
| Sunitinib | 139 (46.5) |
| Sorafenib | 34 (11.4) |
| Axitinib | 1 (0.3) |
| Bevacizumab | 20 (6.7) |
| Temsirolimus | 2 (0.7) |
| Everolimus | 1 (0.3) |
| Others | 15 (5.0) |
| Missing | 12 (4.0) |
| Median duration of treatment with Temsirolimus (months, IQR) | 3.3 (1.5–7.9) |
| Range | 0–49.1 |
| Median dose intensity (IQR) | 89.9% (77.2–100) |
| Discontinued temsirolimus | 424 (77.5) |
| Response evaluation (n=385) | |
| Complete remission (CR) | 2 (0.5) |
| Partial remission (PR) | 53 (13.8) |
| Stable disease (SD) | 194 (50.4) |
| Progressive disease (PD) | 136 (35.3) |
No, number; IQR, inter-quartile range; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Effectiveness by prognostic score and physician’s assessment
The prognosis of the patients was assessed in several ways: MSKCC-, IMDC-, and Hudes scores were calculated from clinical parameters (where possible) and the physician’s individual prognosis forecast by clinical judgement was documented separately. An overview over these data is shown in Table 3.
Table 3
| Variable | No (%) |
|---|---|
| MSKCC risk groups (n=305) | |
| Good prognosis | 7 (1.3) |
| Intermediate prognosis | 153 (28.0) |
| Poor prognosis | 145 (26.5) |
| Unknown | 242 (44.2) |
| Risk groups according to Hudes (n=187) | |
| Non-poor prognosis | 40 (7.3) |
| Poor prognosis | 147 (26.9) |
| Unknown | 360 (65.8) |
| IMDC risk groups (n=248) | |
| Good prognosis | 8 (1.5) |
| Intermediate prognosis | 95 (17.4) |
| Poor prognosis | 145 (26.5) |
| Unknown | 299 (54.7) |
| Subjective prognostic assessment by physician before therapy (n=530) | |
| Good | 50 (9.1) |
| Intermediate | 197 (36.0) |
| Poor | 283 (51.7) |
| Unknown | 17 (3.1) |
No, number; MSKCC, Memorial Sloan-Kettering Cancer Center prognostic score; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium risk score.
The Kaplan-Meier estimates (Figures 1 and 2) showed that the median PFS for the intermediate and the poor prognosis patients for the MSKCC- and the IMDC-score were almost identical with 5.2 months (95% CI: 3.8–6.5 months) vs. 5.6 months (95% CI: 3.8–6.5 months) and 2.5 months (95% CI: 1.8–3.2 months) vs. 2.5 months (95% CI: 1.9-3.2 months), respectively. This was also true for the median OS of the patients: 13.3 months (95% CI: 1.2–15.7 months) vs. 14.2 months (95% CI: 9.4–18.4 months) and 5.5 months (95% CI: 4.3–6.5 months) vs. 5.5 months (95% CI: 4.1–7.1 months), respectively. A comparison of the good prognosis groups by MSKCC and IMDC was not meaningful because of very low numbers of patients, n=7 and n=8, respectively. Additionally, there was no statistical difference between the median PFS of non-poor and poor prognosis patients according to the Hudes-score (P=0.66) and the worst of the log-rank test p-values of all objective scoring systems for the difference of median OS for the Hudes-score (P=0.02). Since the IMDC-Score yielded no additional information compared to the widely used and accepted MSKCC score, we put our focus on the comparison of the MSKCC score and physician’s risk assessment for our further analyses.
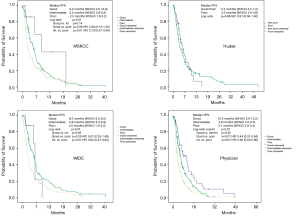
Only 7 out of 305 patients with evaluable MSKCC-score (1.3%) were classified as good prognosis. However, for the physician’s assessment, 50 out of 530 patients (9.4%) were classified as good prognosis (Table 4). For the physician’s assessment, the median PFS of good prognosis patients was 10.4 months (95% CI: 5.6–15.2 months) compared with 5.8 months (95% CI: 5.0–6.5 months) for intermediate and 3.1 months (95% CI: 2.8–3.4 months) for poor prognosis patients. The log-rank p-value for the whole comparison was <0.01. For comparison of good vs. intermediate, good vs. poor and intermediate vs. poor the log-rank p-values were 0.02, <0.01 and <0.01, respectively. For the MSKCC-score analysis of PFS the median survival of the only 7 patients with objective good prognosis was 9.2 months (95% CI: 2.6–15.9 months) and 5.2 months (95% CI: 3.8–6.5 months) for intermediate and 2.5 months (95% CI: 1.8–3.2 months) for poor prognosis patients, respectively. The log-rank P value for the whole comparison was 0.01 and 0.14, 0.08 and 0.01 for the comparisons of good vs. intermediate, good vs. poor and intermediate vs. poor prognosis, respectively.
Table 4
| Outcome-measure | MSKCC | IMDC | Hudes | Physician | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | i | p | g | i | p | n-p | p | g | i | p | ||||
| Median PFS (months) | 9.2 | 5.2 | 2.5 | 6.0 | 5.6 | 2.5 | 5.2 | 3.3 | 10.4 | 5.8 | 3.1 | |||
| Multivariate, HR (95% CI) | 0.30 (0.11-0.83) | 0.69 (0.52-0.92) | 1 (ref) | 0.67 (0.24-1.84) | 0.53 (0.38-0.74) | 1 (ref) | 1.07 (0.68-1.68) | 1 (ref) | 0.45 (0.30-0.66) | 0.64 (0.51-0.80) | 1 (ref) | |||
| P value | 0.02 | 0.01 | 0.43 | <0.01 | 0.78 | <0.01 | <0.01 | |||||||
| Median OS (months) | 34.8 | 13.3 | 5.5 | 9.9 | 13.9 | 5.6 | 12.4 | 7.4 | 25.8 | 14.6 | 7.3 | |||
| Multivariate, HR (95% CI) | 0.26 (0.11-0.85) | 0.54 (0.39-0.75) | 1 (ref) | 0.94 (0.29-3.04) | 0.62 (0.43-0.88) | 1 (ref) | 0.61 (0.36-1.05) | 1 (ref) | 0.41 (0.26-0.65) | 0.55 (0.43-0.71) | 1 (ref) | |||
| P value | 0.03 | <0.01 | 0.92 | <0.01 | 0.07 | <0.01 | <0.01 | |||||||
| n (%) | 7 (1.3) | 153 (50.2) | 145 (47.5) | 8 (3.2) | 95 (38.3) | 145 (58.5) | 40 (21.4) | 147 (78.6) | 50 (9.4) | 197 (37.1) | 283 (53.4) | |||
MSKCC, Memorial Sloan-Kettering Cancer Center prognostic score; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium prognostic score; g, good; i, intermediate; p, poor; n-p, non-poor; PFS, progression-free survival; HR, hazard ratio; 95% CI, 95% confidence interval; OS, overall survival; n, number of patients.
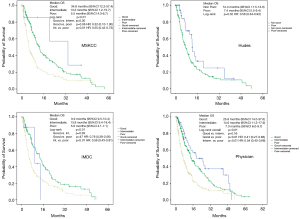
The Kaplan-Meier analysis of OS for patients with good prognosis by judgement of the physician showed a median survival of 25.8 months (95% CI: 14.0–37.6 months) compared with 14.6 months (95% CI: 11.3–17.9 months) for intermediate and 7.3 months (95% CI: 6.0–8.7 months) for poor prognosis, respectively. The log-rank P value for the whole test was <0.01 and for comparison between good vs. intermediate, good vs. poor and intermediate vs. poor prognosis 0.16, <0.01 and <0.01, respectively.
For the MSKCC-score the median OS for good prognosis was 34.8 months (95% CI: 12.2–57.4 months) and for intermediate and poor prognosis 13.3 months (95% CI: 1.2–15.7 months) and 5.5 months (95% CI: 4.3–6.7 months), respectively. Here the log-rank P value for the whole comparison was <0.01 and for the comparison for good vs. intermediate, good vs. poor and intermediate vs. poor prognosis 0.30, 0.06 and <0.01, respectively.
In multivariate analysis, after adjustment for age, gender, histological subtype, number of organ systems with metastases and line of therapy, setting poor risk prognosis as reference, both the MSKCC (HR: 0.60; 95% CI: 0.43–0.84; P<0.01) and the physician prognosis (HR: 0.71; 95% CI: 0.50–0.99; P=0.05) remained independent prognosticators of OS for the intermediate prognosis while for good prognosis only the MSKCC was an independent prognosticator of good survival (HR: 0.30; 95% CI: 0.09–0.98; P=0.05). There was a trend for the physician’s good prognosis towards significance (HR: 0.65; 95% CI: 0.37–1.14; P=0.13).
To assess the ability of the physicians to identify good prognosis patients more deeply, we conducted pairwise comparisons between MSKCC-score and physician’s assessment to evaluate details of the prognostic values for both tools. Table 5 shows the results of these comparisons, Figures 3 and 4 illustrate a graphic representation of our calculations and displays the relevant data for PFS and OS, respectively.
Table 5
| Risk-attribution | Both g | Phys g MSKCC i | Phys g MSKCC p | Phys i MSKCC g | both i | Phys i MSKCC p | Phys p MSKCC g | Phys p MSKCC i | Both p |
|---|---|---|---|---|---|---|---|---|---|
| Both g | 0.40 | 0.14 | 0.32 | 0.11 | 0.25 | 0.26 | 0.20 | 0.12 | |
| Phys g MSKCC i | 0.40 | 0.04 | 0.83 | 0.09 | 0.39 | 0.17 | 0.11 | <0.01 | |
| Phys g MSKCC p | 0.14 | 0.04 | 0.07 | 0.10 | 0.73 | 0.40 | 0.41 | 0.64 | |
| Phys i MSKCC g | 0.32 | 0.83 | 0.07 | 0.29 | 0.31 | 0.16 | 0.26 | 0.09 | |
| Both i | 0.11 | 0.09 | 0.10 | 0.29 | 0.68 | 0.34 | 0.29 | <0.01 | |
| Phys i MSKCC p | 0.25 | 0.39 | 0.73 | 0.31 | 0.68 | 0.59 | 0.86 | 0.09 | |
| Phys p MSKCC g | 0.26 | 0.17 | 0.40 | 0.16 | 0.34 | 0.59 | 0.79 | 0.85 | |
| Phys p MSKCC i | 0.20 | 0.11 | 0.41 | 0.26 | 0.29 | 0.86 | 0.79 | <0.01 | |
| Both p | 0.12 | <0.01 | 0.64 | 0.09 | <0.01 | 0.09 | 0.85 | <0.01 |
MSKCC, Memorial Sloan-Kettering Cancer Center prognostic score; g, good; i, intermediate; p, poor; n-p, non-poor.
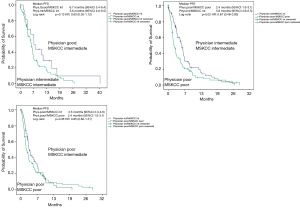
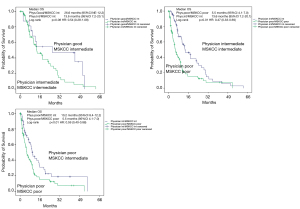
In patients with good prognosis attributed by the physician and at the same time intermediate prognosis assigned by MSKCC, the median overall survival was nearly doubled compared to consensual intermediate prognosis (26.6 vs. 13.6 months), albeit without reaching statistical significance (P=0.08). If MSKCC and physician’s assessment were consensual, statistical significance was given for differentiation between intermediate and poor prognosis pts (P<0.01). In patients with poor prognosis assessed by the physician, MSKCC performed statistically better for differentiation between poor and intermediate prognosis with a median OS of 10.3 vs. 5.5 months (P<0.01).
Discussion
Locally advanced/inoperable or metastatic renal cell cancer can nowadays be treated with a growing multitude of drugs. The prognosis of different patient subgroups differs considerably. Prognostic scores using objective parameters like hemoglobin level, LDH level and others can be used to assign the patients to different prognostic subgroups (good, intermediate and poor prognosis). Currently, the most accepted objective prognostic scoring system are the MSKCC-score (13,14), the IMDC-score (15) and, for the therapy with TEMS, the Hudes-Score (5,13). These scores can then be used to make treatment decisions and assign respective therapies to the patients within their respective position in the sequence of the a/mRCC treatment armamentarium. The ESMO-guidelines (16) or the NCCN-guidelines (17) make evidence-based suggestions which medication to apply using IMDC- or MSKCC-criteria. In real world medicine however, there are more parameters than the objectively measurable ones used in the MSKCC or IMDC-score that a physician has to take into account. For example, there are parameters like general appearance, appetite, fatigue and many more that an experienced physician uses to make his own prognostic assessment of his patients. This prospective multicenter phase 4 registry study gave the unique opportunity to compare the objective prognostic scores with the subjective assessment of the physician and to identify subsets of patients which may be better assessed by the respective prognostic methods.
We found that either prognostic tool results in significant and clinically meaningful differentiation between good, intermediate, and poor prognosis patients. However, it is obvious that by physician’s risk assessment considerably more patients can be attributed to good prognosis than by the MSKCC-score (9.1% vs. 1.3%). These patients have a significantly longer survival than those patients identified by the physicians as being of intermediate prognosis.
To assess this further, we conducted pairwise comparisons between MSKCC score and physician’s assessment to evaluate details of the prognostic values for both tools. In patients with good prognosis attributed by the physician and intermediate prognosis assigned by MSKCC, the survival was nearly doubled compared to consensual intermediate prognosis, albeit without reaching statistical significance (P=0.08). However, we still consider this finding clinically meaningful. If MSKCC and physician’s assessment were consensual, statistical significance was given for differentiation between intermediate and poor prognosis patients. In patients with poor prognosis assessed by the physician, MSKCC performed statistically better for differentiation between poor and intermediate prognosis. Here, the group of patients with poor prognosis by the physician and intermediate prognosis by MSKCC-score had a twice as long survival than the group of patients with consensual poor prognosis by physician and MSKCC-score. Thus, the physicians additionally interpreting soft parameters as general appearance, appetite and more seem to be able to identify a subset of patients with good prognosis that the MSKCC-score may have falsely assigned to intermediate prognosis and the MSKCC-score calculating hard parameters like hemoglobin or LDH-levels seems to be able to identify patients with true poor prognosis which the physician may have falsely assigned to intermediate prognosis. These findings may have importance for clinicians having to make treatment decisions in a/mRCC patients.
In other diseases, prognostic scores are used as well. While most of the scoring and indexing systems use hard and objective criteria (20-25), some, including soft and rather subjective factors, seem to ad helpful information. For example, in ovarian cancer the IOTA logistic regression-model comprising several objective factors like cancer antigen 125 (CA-125) and family history but also softer factors like the highly physician dependent pelvic ultrasound including Doppler sonography seemed to be superior to other prognostic scores with objective factors only like the “risk of ovarian malignancy”-algorithm which includes the levels of CA-125 and human epididymis protein 4 (HE4) (25). For advanced colorectal cancer, the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ)-30 was found to be independently prognostic for OS when applied at baseline before treatment with either sequential treatment with capecitabine, irinotecan and capecitabine plus oxaliplatin or upfront combination treatment with capecitabine plus irinotecan followed by capecitabine plus oxaliplatin (22). Since questionnaires try to objectively assess subjective factors like in this case quality of life factors which in the case of our study was done with the “gut feeling” of the physician it may be worthwhile to examine standardized questionnaires combined with for example the MSKCC-score. To take this into account the StarTOR registry was amended with the inclusion of quality of life questionnaires.
Our study is not without limitations. Due to its non-interventional design, response and progression were assessed by the attending physician and not by an independent review. The staging intervals were not predefined but were between 2 und 3-monthly in nearly all of the patients. Due to lack of baseline parameters the MSKCC could not be calculated in all of the patients treated with TEMS and registered in the StarTOR registry. However, this represents the so far largest prospective population of patients treated with TEMS and therefore we think that our finding may be meaningful for the community of physicians treating kidney cancer patients. Further, with the recent advances in systemic treatment of kidney cancer using therapy regimen involving immunotherapy, TEMS is no more the treatment of choice in the majority of patients. Still, for patients not able to receive immunotherapy because of intraindividual reasons or just because of non-availability of immunotherapy in regions of the world, we believe our findings are of importance for patients and physicians in these situations. Further studies like the present will have to show if this concept will be adaptable to other therapies in a/mRCC patients.
Conclusions
The STAR-TOR registry offered the rare opportunity to compare prognostic scores calculated from objective values with the prognosis estimation given by the treating physician using additional subjective information. In this comparison, the physician performed rather well and was obviously able to identify a subset of patients with a good prognosis out of the MSKCC-intermediate group while the MSKCC score could identify patients which were falsely placed in the poor risk group by the physician. Thus, in daily practice the practitioner may consider these findings in patients with intermediate prognosis based on the MSKCC score and poor prognosis based on his experience before attributing a therapy for his patient.
Further investigations have to be done to verify this hypothesis.
Acknowledgments
The authors would like to thank all participating sites involved in the STAR-TOR registry.
Funding: The STAR-TOR registry is funded by Pfizer. The present analysis is exploratory and was done independently from Pfizer.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tilman Todenhöfer) for the series “Management of Advanced Genitourinary Malignancies” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-938
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-938). The series “Management of Advanced Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. Dr. MB reports personal fees from Pfizer, during the conduct of the study; grants and personal fees from Janssen, personal fees from Astellas, personal fees from Bayer, personal fees from AstraZeneca, personal fees from Sanofi, personal fees from Pfizer, personal fees from Novartis, personal fees from EUSApharm, personal fees from Amgen, personal fees from Ipsen, personal fees from Merck, personal fees from MSD, personal fees from BMS, personal fees from Eisai, personal fees from ABX, outside the submitted work. Dr. PJG reports to have received honoraria/support as a speaker from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Novartis, Pfizer, Roche, Sanofi and to have received honoraria for participation in expert rounds from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Novartis, Pfizer, Roche, Sanofi. Dr. MW is an employee of Pfizer Pharma GmbH, Germany. Dr. JB is an Employee of Pfizer Pharma GmbH, Germany. Dr. KS reports other from Pfizer, during the conduct of the study; personal fees from Janssen, non-financial support from Astellas, non-financial support from Bayer, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Novartis, personal fees from EUSApharm, personal fees from Amgen, personal fees from Ipsen, personal fees from Merck, personal fees from MSD, personal fees from BMS, personal fees from Eisai, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Medical Board of Westfalia-Lippe and the Westfalian Wilhelms-University in Muenster (Approval number: 2007-484-f-S) and all patients had given written informed consent prior to any study-specific actions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1814-23. [Crossref] [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009;27:3312-8. [Crossref] [PubMed]
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103-11. [Crossref] [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473-82. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [Crossref] [PubMed]
- Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791-9. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [Crossref] [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40. [Crossref] [PubMed]
- Patil S, Figlin RA, Hutson TE, et al. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol 2011;22:295-300. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019;30:706-20. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines for Patients®: Kidney Cancer. Available online: https://wwwnccnorg/patients/guidelines/content/PDF/kidney-patientpdf. 2020.
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:804-34. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Gray EP, Teare MD, Stevens J, et al. Risk Prediction Models for Lung Cancer: A Systematic Review. Clin Lung Cancer 2016;17:95-106. [Crossref] [PubMed]
- Merkel S, Bialecki D, Meyer T, et al. Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol 2009;100:349-57. [Crossref] [PubMed]
- Mol L, Ottevanger PB, Koopman M, et al. The prognostic value of WHO performance status in relation to quality of life in advanced colorectal cancer patients. Eur J Cancer 2016;66:138-43. [Crossref] [PubMed]
- Prosperi MC, Ingham SL, Howell A, et al. Can multiple SNP testing in BRCA2 and BRCA1 female carriers be used to improve risk prediction models in conjunction with clinical assessment? BMC Med Inform Decis Mak 2014;14:87. [Crossref] [PubMed]
- Rodrigues G, Gonzalez-Maldonado S, Bauman G, et al. A statistical comparison of prognostic index systems for brain metastases after stereotactic radiosurgery or fractionated stereotactic radiation therapy. Clin Oncol (R Coll Radiol) 2013;25:227-35. [Crossref] [PubMed]
- Stukan M, Dudziak M, Ratajczak K, et al. Usefulness of diagnostic indices comprising clinical, sonographic, and biomarker data for discriminating benign from malignant ovarian masses. J Ultrasound Med 2015;34:207-17. [Crossref] [PubMed]


