Modifying sunitinib schedule in advanced kidney cancer patients: Reflections from the results of the renal EFFECT trial
On March 19th 2012, the results of the Renal EFFECT trial were finally published ahead of print by the authoritative Journal of Clinical Oncology (1).
The Renal EFFECT trial was a randomized phase II trial of sunitinib given to advanced renal cell carcinoma (RCC) patients, either according to the standard schedule (50 mg daily, 4 weeks on, 2 weeks off) or according to a modified schedule, with sunitinib given continuously at the reduced dose of 37.5 mg daily.
Even though the declared primary end-point of the study was time to progression (TTP), and despite authors claimed that “this trial was not designed to be either a superiority or a noninferiority trial” (1), in practical terms the study was supposed to answer a completely different question, i.e., is the continuous dosing schedule equieffective, but safer, as compared to the standard schedule?
Let’s start to analyze the results of this study in terms of efficacy.
Median TTP was 9.9 months for the classical schedule vs. 7.1 months for the continuous daily dose schedule; consistent with the TTP analysis, a longer progression-free survival (PFS) was observed in patients treated with the classical schedule: 8.5 vs. 7.0 months; overall survival, on the other hand, was almost superimposible between the two treatment arms (23.1 vs. 23.5 months) (1).
As a whole, the observed better performance in terms of efficacy outcome measures (TTP and PFS) of the classical schedule is someway in agreement with a recent pharmacokinetic/pharmacodynamic meta-analysis aimed at investigating the relationship between sunitinib exposure and efficacy and tolerability endpoints (2); according to such meta-analysis, the importance of maintaining patients on a 50 mg dose of sunitinib and striving to avoid unscheduled dose titrations (as well as unscheduled treatment interruptions) during treatment, clearly emerged (2). Indeed, patients with the highest exposure to sunitinib displayed longer TTP, longer OS, a higher probability of a response, and greater tumor size decreases (2).
Despite that, the absolute PFS values observed in the two treatment arms of the renal EFFECT trial (1) were someway disappointing; no one would infact consider the 8.5 months of PFS achieved by the standard arm a satisfactory outcome for first line sunitinib, irrespective of any statistical consideration.
And indeed, all the efficacy figures reported in the renal EFFECT trial were lower than those accomplished by sunitinib (given according to the standard schedule) within the pivotal registration trial, as summarized in Table 1 (3).
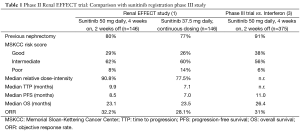
Full table
These results, however, are probably relatively easy to explain. Indeed, we agree with study investigators that “… is not an unusual observation, when progressing from more highly selective pivotal phase III efficacy trials to subsequent effectiveness studies with broader elegibility criteria …”, to sometime observe a relevant drop in efficacy measures (1).
Furthermore, as again stressed by the authors in their discussion, at the time of the conduction of this trial, a range of treatment options were already available, inevitably leading to a selection bias, not to take into account the possible temptation to switch therapy early (1), instead of trying to optimize treatment adequately, and aggresively managing adverse events to keep patients on treatment.
The safety profile of the two schedules is a completely different, and more complex, issue.
Indeed, the study showed no significant between-arm differences in the incidence of any grade 3 or 4 adverse events, or of any grade 3 or 4 laboratory abnormalities; furthermore, as far as the rate of treatment discontinuations due to adverse events, it was 11% and 15% in the classical and continuous dosing schedule, respectively, another surprising (and someway unexpected) finding (1). Finally, a superiority of the standard schedule over the modified one in time to deterioration (i.e., a composite end-point of death, progression and disease-related symptoms) was observed (1).
For sure, the lack of the two weeks’ rest in the modified schedule have played a role, not allowing an adequate recovery from sunitinib-related adverse events. Furthermore, as already clearly evidenced from everyday clinical practice, multiple and prolonged nonsevere toxicities may lead to a more deleterious impact on patients’ quality of life, than a single, severe, but short-term, toxicity.
At this point, a key question remains unanswered: how to ameliorate the safety profile of sunitinib, to reduce unnecessary (and possibly detrimental) dose reductions and treatment interruptions?
A population pharmacokinetic analysis of data from studies performed in healthy volunteers and patients with cancer treated with sunitinib clearly showed a high inter-patient variability in pharmacokinetics, with coefficients of variation in the range of 40-60%, meaning that certain patients in a given population treated with the same dose/schedule may experience increased exposure to sunitinib. For example, it has been calculated that approximately 8% of patients given sunitinib 50 mg QD would have at least as much exposure (AUC) as a typical individual receiving a 75 mg QD dose (4). Furthermore, this population pharmacokinetic analysis identified female gender and low body weight as covariates that significantly increase exposure to sunitinib (4).
An interesting report from a Dutch group already raised the issue that sunitinib dosing schedule (the classical 50 mg daily, 4 weeks on, 2 weeks off) could be suboptimal for unselected mRCC patients; indeed, a number of patients are initially overtreated resulting in unnecessary adverse events, while other patients who do not experience any toxicity may be undertreated (5). With the use of the fixed dosing regimen, similarly to the population pharmacokinetic study addressed above, the authors found a highly significant correlation between severe sunitinib-related toxicity and patient characteristics such as BSA, female gender, and high age (5).
We cannot but agree with the authors’ conclusions that attempts to optimise the dosing schedule of sunitinib in unselected metastatic RCC patients are warranted (5).
As a whole, all the above data clearly suggest that the modified schedule used in the renal effective trial did not achieve the goal of being better tolerated and equieffective, as compared to the standard schedule, and that the idea itself of giving sunitinib (and perhalps also all the other targeted agents presently used in RCC) at a fixed dose makes little sense, if any.
Indeed, alternative schedules and dosing (e.g., on the basis of BSA) should be pursued, but will it be so?
Probably not, unfortunately, thus leaving patients experiencing unnecessary toxicities and detrimental dose reductions and treatment discontinuations.
Acknowledgements
None.
Footnote
Conflicts of Interest: Camillo Porta acted as a consultant and/or speaker for Pfizer Oncology, GSK, Hoffman La Roche, Bayer-Schering Pharma, Novartis Pharma, Aveo Pharmaceuticals, Astellas, Boehringer Ingellheim and Recordati. Chiara Paglino acted as a speaker for Pfizer Oncology, GSK, Hoffman La Roche and Bayer-Schering Pharma. Carlo Ganini has no conflicts of interest to declare.
References
- Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of Sunitinibon an intermittent versus continuous dosing schedule as first-line therapy for advanced Renal Cell Carcinoma. J Clin Oncol 2012;30:1371-7. [PubMed]
- Houk BE, Bello CL, Poland B, et al. Relationship between exposure to Sunitinib and tolerability endpoint in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357-71. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon alfa in metastatic renal cell carcinoma. N Engl J Med 2007;356:115-24. [PubMed]
- Houk BE, Bello CL, Kang D, et al. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009;15:2497-506. [PubMed]
- van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer 2008;99:259-65. [PubMed]


