Sequence of treatment in locally advanced and metastatic renal cell carcinoma
Introduction
Generally, in the treatment of solid tumours, the most effective drug, which provides the best response rate (RR), progression free survival (PFS) and possibly overall survival (OS), is the treatment of choice in the first-line setting. However, there are exceptions to this rule and concessions are made, especially when toxicity concerns come into play, e.g., in elderly patients (1), or when disease stabilization (SD) is a valid treatment objective (2). The fundamental dichotomy in solid tumour oncology of tumour response, a time-tested marker of therapeutic efficacy, and disease progression, an essential sign of treatment failure, has recently been challenged (3). Randomized clinical trials assess new treatments in comparison with established therapies in superiority or non-inferiority studies and are aimed to establish a position in the hierarchy of available treatments.
The concept of sequencing treatment is relatively new and there is little literature on this topic in solid tumours. Sequencing therapies may be discussed as a distinction from combining treatments, with examples in colorectal cancer (CRC) (4,5) and a Cochrane review in breast cancer (6). Alternatively, sequencing may be evaluated in the context of treatment order (7) or as a combination of both questions (8). The prerequisites for discussing the sequence of anti-cancer treatment are the availability of several active drugs and the indication that certain treatments may be more or less active before or after another. This alludes to the topic of drug resistance and overcoming resistance mechanisms (9).
Colorectal cancer
In CRC only one randomized trial compared folinic acid, 5-fluoruracil, and irinotecan (FOLFIRI) followed by folinic acid, 5-fluoruracil and oxaliplatin (FOLFOX) or the reverse; however both sequences FOLFIRI → FOLFOX and FOLFOX → FOLFIRI achieved a prolonged survival and similar efficacy (10). The fact that a substantial proportion of patients (26% and 38%) did not receive second-line therapy demonstrated the importance of the choice in first-line therapy.
Prostate cancer
Many new treatments have recently been assessed and licensed in metastatic castration resistant prostate cancer (CRPC) (11-16) and sequence of drugs has become an issue (17). Studies indicate that CRPC with acquired resistance to first-generation androgen receptor (AR) inhibitors maintain reliance on AR signalling for survival (18) and are sensitive to subsequent therapy with second-generation AR inhibitors such as enzalutamide. However, the glucocorticoid receptor confers resistance to AR inhibitors by bypassing AR blockade and mediates enzalutamide resistance. This novel mechanism of escape from AR blockade through expansion of cells primed to drive AR target genes via an alternative nuclear receptor upon drug exposure may therefore be relevant for drug sequencing (19). In contrast, prior treatment with the androgen synthesis inhibitor ketoconazole did not have an impact on the clinical outcomes of patients with CRPC who received subsequent docetaxel-based therapy (20,21).
Renal cell carcinoma
The introduction of sorafenib as first targeted therapy in metastatic renal cell carcinoma (RCC) (22) was the beginning of a rapid process which led to the development of other vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKI), the monoclonal VEGF-directed antibody bevacizumab and mammalian target of rapamycin (mTOR) inhibitors for the treatment of locally advanced and metastatic RCC. Crucial for this progress was the understanding of the role of angiogenesis in general and the VEGF- and mTOR-pathways (23). Currently, seven drug or drug combinations are licensed for the treatment of metastatic RCC. Several guidelines recommend targeted agents as standard treatment for metastatic RCC (24-26). The sequence for using these therapies is an ongoing matter of debate and several reviews have been published on this topic over the past years (27-31).
Sequential treatment in RCC is of interest as complete responses (CR) to treatment with TKIs are rare and TKIs usually do not produce long term remissions: patients relapse when therapy is discontinued (32,33), and resistance to treatment inevitably develops during therapy (34). However, the life expectancy of RCC patients has been extended to over 30 months (35) from 13 months in the cytokine era (36). Until 2004 treatment options for RCC were limited and usually immunotherapy was used: interleukin-2 (IL-2) (37) and interferon-alpha (IFN-α) (38), or a combination of both (39,40), depending on patient characteristics, availability of drugs and familiarity with the toxicity management (41). Outcome for the majority of patients was poor (42).
The sequencing question becomes relevant when multiple treatments are developed in a short period of time and new drugs are licensed before others have found a definite place in the armamentarium of therapies. It also gains importance when no direct comparison of drugs is possible due to the delay from trial conception to publication: new drugs become available while others are being evaluated in studies. Sequencing is especially relevant when prior treatment with a certain agent compromises efficacy of a subsequent therapy or enhances the treatment effect.
Some patients will receive several lines of treatment and will obtain a repeated treatment response or at least stable disease. In these patients the order of treatments may be of less relevance compared to other patients, who have aggressive and rapidly progressing disease and need treatment with the most active drug at the beginning. Debating treatment sequence is ultimately an expression of a success story with an embarrassment of riches in the treatment of RCC (43).
In this review we focus on clear cell RCC owing to the fact that only limited data is available on the treatment of patients with non-clear cell RCC and the optimal treatment remains unclear (44).
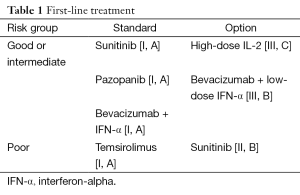
First-line treatment (Table 1)
Full table
In the first-line treatment of metastatic RCC five drugs and drug combinations are currently licensed. Two pivotal trials assessing the TKI sunitinib (45) and the combination of bevacizumab and IFN-α (46) in comparison to standard IFN-α treatment in patients with Memorial Sloan Kettering Cancer Center (MSKCC) favourable and intermediate risk (47) were published in 2007. A concurrent three arm randomized trial evaluated the mTOR inhibitor temsirolimus as single agent compared to temsirolimus in combination with IFN-α compared to IFN-α as single agent in MSKCC poor risk patients (48). While the mTOR inhibitor was demonstrated to prolong OS, the VEGFR-targeting agents showed statistically significant improvement in PFS, which was the primary endpoint of these trials. The median OS of 26.4 months with sunitinib (49) and 23.3 months with bevacizumab and IFN-α (50) were unprecedented at the time. Rini et al. performed a CALGB trial with bevacizumab and IFN-α compared to IFN-α, which produced similar results (51,52) as the European trial.
The multi-TKI pazopanib was first tested in a randomized placebo-controlled phase III trial, with 54% treatment naive and 46% cytokine pre-treated patients (53,54). Due to the promising activity, and the favourable toxicity profile, a cross-over trial assessing treatment preference for pazopanib versus sunitinib was performed (55). The results were published a few months prior to data on treatment efficacy from a non-inferiority trial (56). In summary, pazopanib and sunitinib were found to be equally effective in terms of PFS, RR and OS (57), while quality-of-life favoured pazopanib. Despite the favourable safety and quality-of-life profiles for pazopanib relative to sunitinib, treatment was discontinued due to adverse events in 24% of patients on pazopanib compared to 20% on sunitinib. There is also concern on the validity of the non-inferiority design, given that results of the intention-to-treat analysis differed from the per-protocol analysis (58).
The randomized phase III trial with tivozanib, a potent and selective VEGFR-TKI with a relatively long half-life, failed to show an improvement in OS despite prolonged PFS for tivozanib compared to sorafenib (11.9 vs. 9.1 months) in a mixed population of treatment naïve and cytokine pre-treated patients. Median OS reached 29.3 with sorafenib and 28.8 months with tivozanib, respectively (59). The authors postulate that differential use of second-line therapies confounded OS. They hypothesize that the trend toward longer OS in the sorafenib arm compared to tivozanib is related to the greater proportion of patients in the sorafenib arm who received second-line targeted treatment (63% vs. 13%). In addition, the one-way cross-over design allowed patients who had progressed on sorafenib to switch to tivozanib (61%). In essence, this is a sequential trial of two agents (sorafenib → tivozanib) compared with one agent (tivozanib) (60). Important in the context of sequencing treatments: two consecutive targeted agents are associated with a longer OS than treatment with only one line of targeted therapy (61) and absence of PD after first and second-line targeted therapy may characterize long-term survival (62). An alternative hypothesis to explain the trend toward longer OS on the sorafenib arm is that sorafenib is more effective than tivozanib for improving OS (63). This would not have been expected, since the first-line comparison of sorafenib versus IFN-α demonstrated comparable PFS for the two agents, however no OS data was published (64).
Another trial comparing first-line treatment with the potent and selective second-generation VEGFR inhibitor axitinib and sorafenib was performed in Asian patients. Sorafenib was chosen as the comparator because it was available in the regions where the trial was performed (65). Somewhat surprisingly, the trial was negative and axitinib did not significantly improve PFS (10.1 months) vs. sorafenib (6.5 months). An accompanying comment proposes that no significant difference in efficacy was shown because the study was underpowered and the benefit of sorafenib might have been underestimated (66). The striking difference in outcome for Eastern Cooperative Oncology Group performance status (ECOG) 0 (7.1 months difference in median PFS with axitinib vs. sorafenib) and ECOG 1 (no difference in PFS) might be attributed to the fact that the majority of patients was recruited in Eastern Europe, where resource limitations and local practice standards may have affected the type of patient enrolled, or patient management.
In our view, neither single agent IFN-α (36) nor subcutaneous IL-2 play a role in the treatment of RCC nowadays. This is especially relevant for patients with MSKCC intermediate or poor risk, due to the significant toxicity in these patients (40). However, infusional IL-2 is a treatment option in selected patients and centres, considering the long term survival of some RCC patients on this therapy (67).
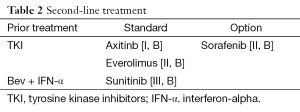
Second-line treatment (Table 2)
Full table
There are four important phase III trials in the second-line setting of RCC. Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) tested treatment with sorafenib versus placebo in patients who were progressing on standard therapy. At the time, standard therapies were mainly cytokines: most patients had received IL-2, IFN-α, or both before progression and enrolment. Nine hundred and three patients were randomized; primary end point of the trial was OS. The first PFS analysis revealed a significant benefit for sorafenib with a PFS of 5.5 vs. 2.8 months for the placebo group. Following these results, study-group assignments were to offer sorafenib to all patients in the placebo group. OS analysis showed a tendency towards longer survival for treatment with sorafenib. However, statistical significance was not reached, mainly due to cross-over from placebo to sorafenib (68). Secondary analysis, censoring post-cross-over placebo survival data, reached statistical significance showing better OS for patients treated with sorafenib (69).
The RECORD-1 (renal cell cancer treatment with oral RAD001 given daily) trial compared everolimus to placebo in RCC patients pre-treated with sunitinib, sorafenib or both. Median PFS was significantly longer with 4.9 months for patients treated with everolimus compared to 1.9 months for patients randomized to receive placebo (70,71). Approval of everolimus was based on the results of the trial. A criticism can be made that this trial was not a pure second-line study. In fact, most patients had received more than one previous treatment-line, including IFN-α, IL-2 and bevacizumab. Twenty-six percent of patients in both treatment arms had been pre-treated with two VEGF-TKIs, namely sunitinib and sorafenib. Therefore, one may accept this trial as a rationale to consider everolimus as third-line option after treatment with two lines of anti-VEGF directed therapy. It is noteworthy that subgroup analysis revealed a benefit for patients in the everolimus arm who were pre-treated with only one VEGF-TKI compared to those pre-treated with two previous VEGF-TKIs (PFS 5.4 months for everolimus vs. 1.9 months for placebo after one previous VEGF-TKI; PFS 4.0 months with everolimus vs. 1.8 months with placebo after two previous VEGF-TKIs) (72).
There are two randomised phase III trials comparing different VEGF-TKIs and VEGF-TKI versus an mTOR inhibitor in the second-line, respectively.
The AXIS (comparative effectiveness of axitinib versus sorafenib in advanced RCC) trial randomized 723 patients who had progressed after first-line treatment with sunitinib, bevacizumab plus IFN-α, temsirolimus or cytokines to receive axitinib or sorafenib in the second-line. PFS was significantly longer for patients assigned to axitinib (6.7 vs. 4.7 months for sorafenib) (73). Although OR rate was also significantly better for axitinib, no significant OS benefit could be shown (74).
In the INTORSECT (Investigating Torisel As Second-Line Therapy) trial, patients who had progressed after treatment with sunitinib were randomized to receive the mTOR inhibitor temsirolimus or the TKI sorafenib. Five hundred and twelve patients were included and stratification according to duration of prior sunitinib therapy was performed. Although no significant difference in PFS was observed, OS was significantly longer for patients treated with sorafenib compared to those treated with temsirolimus (16.6 vs. 12.3 months). Subgroup analysis showed that median OS with sorafenib was only longer in comparison to temsirolimus for patients whose duration of pre-treatment with sunitinib was >180 days (17.8 vs. 14.4 months). For patients responding <180 days to sunitinib, no significant difference was observed (11.4 months for sorafenib vs. 10.1 months for temsirolimus) (75). Interpreting these results, one may assume that patients, who responded to anti VEGF-therapy in the first-line, should receive a VEGF-TKI in second-line. However, subgroup analysis should always be interpreted with caution and as OS is generally shorter in both treatment arms for patients with sunitinib response <180 days, one may also conclude that patients showing little benefit from first-line VEGF-TKI generally have a worse prognosis.
There is a phase II study analysing antitumour activity of sunitinib in patients pre-treated with bevacizumab. Twenty-three percent of patients showed a PR with sunitinib, and SD as best response was seen in 59% of patients. Median OS was 47.1 weeks (76). These data support the assumption of clinical benefit from sequential anti-VEGF directed therapy in patients with advanced and metastatic RCC.
Third-line treatment (Table 3)

Full table
In 2015 only limited data exist for the choice of third-line treatment in patients with metastatic RCC. Treatment selection is based on the treating physician’s individual experience and availability of drugs rather than on scientific evidence.
An Italian retrospective study (77) analyzed sorafenib as third-line treatment after sequential therapy with sunitinib and mTOR inhibitors (everolimus or temsirolimus). A total of 34 patients were included. Median PFS was 4 months, and median OS 7 months. There were no treatment interruptions due to toxicity. Response to sorafenib was better in patients who had already responded to sunitinib in the first-line whereas no activity was seen in patients without previous benefit from sunitinib.
Although not directly comparable, one may assume that a median PFS of 4 months with third-line sorafenib is a sign of drug activity, when taking data from the RECORD-1 trial into consideration: patients receiving placebo had a median PFS of only 1.9 months (70). Most of these patients had received more than one previous treatment-line. Therefore one may presume that sorafenib is superior to placebo in the third-line setting.
So far, only one randomized prospective trial concerning third-line therapy in patients with metastatic RCC has been conducted (78). Patients who had failed previous treatment with one VEGF-targeted therapy and one mTOR inhibitor were randomized to receive either sorafenib or the VEGF and fibroblast growth factor (FGF) receptor inhibitor dovitinib. The rationale for selecting dovitinib was derived from the hypothesis that adaptive resistance to anti-VEGF therapy may be caused by activation of the FGF pathway (79). Therefore, it was hypothesized that such a mechanism could be overcome by adding a TKI with FGF inhibiting properties. However, no differences regarding PFS or OS were observed between the two treatment arms.
In a small retrospective analysis, 40 patients with everolimus resistant RCC were treated with a VEGFR-TKI (80). All patients had received first-line VEGF-targeted therapy (sunitinib, sorafenib or bevacizumab and IFN-α) and this was associated with a median PFS of 11.3 months. A subset of ten patients was treated with a second-line TKI. Treatment with everolimus was associated with a median PFS of 5.9 months. Subsequent treatment after everolimus was associated with SD in 22 patients (55%) and PR in 4 patients (10%), whereas eleven patients had PD (28%). The median PFS on therapy after everolimus failure was 5.5 months. This data suggests that VEGF-resistance remains transient in nature, at least in initially susceptible patients.
Re-challenge
Based on the hypothesis that re-challenge of patients with a previously used VEGF-targeting agent could be a rationale strategy for tumour control, a retrospective review was undertaken to describe the experience of re-challenge with sunitinib in metastatic RCC (81). The investigators identified 23 patients who were re-challenged with sunitinib. The initial median PFS among these patients had been 13.7 months and was in line with the registration study (45). At re-challenge, median PFS was 7.2 months. Upon re-challenge, 22% of patients achieved a PR, while 74% had SD as their best response. Patients with a >6 months interval between sunitinib treatments had better PFS with re-challenge compared to patients who started the re-challenge within 6 months of discontinuing their initial treatment.
In another study, the efficacy of sunitinib re-challenge was assessed in two German centres. Thirteen patients received sunitinib (median PFS 21 months) and were subsequently treated with an mTOR inhibitor; upon disease progression they received sunitinib again. This approach resulted in a median PFS of 6.9 months and consisted of two (15%) PR and ten (77%) SD (82). These two retrospective analyses and several case series serve as a proof of concept and have just recently been summarized and discussed in a review article (83).
Twelve patients who had previously received VEGF-targeted treatment and an mTOR inhibitor were re-challenged with a second mTOR inhibitor. Both sequences everolimus → temsirolimus (n=7) and temsirolimus → everolimus (n=5) were used. Six of 12 patients (50%) responded to everolimus and four of 12 patients (33%) responded to temsirolimus, however only one patient responded to both agents and three patients to none. Median treatment duration for everolimus → temsirolimus and temsirolimus → everolimus sequences were 10.3 and 5.8 months, respectively (84). No patient responded to temsirolimus re-challenge after response to everolimus as the first mTOR inhibitor, whereas patients who did not respond to everolimus as the first mTOR inhibitor may still respond to a re-challenge with temsirolimus (2/7). Despite structural similarities of both mTOR inhibitors and the same mode of action, the two drugs have distinct clinical pharmacokinetic and pharmacodynamic profiles, which may contribute to differing responses in patients. Due to the small sample size no definitive conclusions can be drawn from this data. In settings where several drugs are available, re-challenge is of limited interest. However, in countries with less treatment choices this topic might still be of relevance.
Trials assessing sequential treatment
There is only one randomized trial assessing a treatment sequence in RCC. In this phase II trial 471 patients were either assigned to first-line mTOR inhibitor everolimus followed by the TKI sunitinib upon disease progression (everolimus → sunitinib), or fist-line sunitinib followed by everolimus (sunitinib → everolimus) (35). Only 45% and 43% of the patients crossed-over and received second-line treatment, respectively. The primary endpoint, PFS non-inferiority of first-line everolimus compared with first-line sunitinib, was not met: the median PFS was 7.9 months for first-line everolimus and 10.7 months for first-line sunitinib. The median combined PFS was 21.1 months for everolimus → sunitinib and 25.8 months for sunitinib → everolimus. Feasibility for the combined PFS end point had not been established previously. Median OS was longer for sunitinib → everolimus (32 months) compared to everolimus → sunitinib (22.4 months). The hypothesis of the investigators that similar combined PFS lengths would be achieved by both sequences and that everolimus would be better tolerated than sunitinib as the first-line therapy was not confirmed.
In a retrospective French analysis, outcome of patients with either sunitinib followed by sorafenib (sunitinib → sorafenib) or sorafenib followed by sunitinib (sorafenib → sunitinib) was assessed (85). Of note, the majority of the 90 patients had received prior cytokines. The treatment durations were 61 weeks for sorafenib → sunitinib (33 weeks → 28 weeks) and 49 weeks for sunitinib → sorafenib (27 weeks → 22 weeks), respectively. These data confirm absence of absolute cross-resistance between sunitinib and sorafenib. They do not, however, guide on the optimal treatment sequence, especially in patients without prior cytokine exposure.
Biological aspects
Discussing optimal treatment strategies for advanced and metastatic RCC demands a closer look at biological aspects underlying this disease. Inactivation of the von Hippel Lindau (VHL) gene, a tumour suppressor gene, is crucial in the development of the disease. VHL encodes a protein, which supports degradation of hypoxia-inducible factor (HIF). Inactivation of VHL therefore leads to higher levels of the transcription factor HIF, which promotes transcription of several genes such as VEGF, platelet-derived growth factor (PDGF) and transforming growth factor alpha (TGF-α) (86,87). These are important factors for angiogenesis. Induction of chronic angiogenesis is crucial in the development of cancer and has been described as one of the “hallmarks of cancer” (88). RCC is a highly vascularized tumour type, thus targeting angiogenesis seems a promising treatment strategy.
However, some patients are primarily resistant to these targeted treatments and almost all show tumour progression over a longer period of time, even if there has been a tumour response to treatment in the beginning.
In patients with lack of response to VEGF-targeted therapy, primary resistance needs to be differentiated from inadequate dosing. TKIs can cause a diversity of adverse events such as hypertension or hand-foot-syndrome, which may lead to dose modifications due to intolerable toxicities. Moreover, most cancer patients are of older age and receive co-medication with several other drugs. This bears potential for cytochrome P interactions and inadequate drug exposure. Animal models (89) and a meta-analysis (90) could show that increased exposure to sunitinib is associated with improved clinical outcomes and that increasing sunitinib dose can partly overcome resistance in xenografts and patients. There are two phase II trials assessing feasibility of dose escalation in patients treated with sorafenib (91). Some patients obtained a response upon dose escalation after early progression to standard dose (92). Axitinib dose titration in previously untreated patients was evaluated in a randomized phase II trial against placebo titration, as retrospective population pharmacokinetic data suggest axitinib plasma exposure correlates with efficacy in metastatic RCC (93). In fact, the greater proportion of patients in the axitinib titration group achieving an OR supports the concept of individual axitinib dose titration (94).
Taken together, adequate dosing of the antineoplastic drug and optimal management of potential side effects should be ensured before treatment strategy is changed due to suspected resistance, especially if tumour response has been observed in the beginning and dose reductions have taken place.
The challenge of adherence has been recognized in oncology practice (95). However, limited data is available on adherence to targeted therapies and efforts towards better patient education are warranted including dedicated staff for monitoring outpatient anticancer oral therapy (96).
PD is often defined by Response Evaluation Criteria in Solid Tumors (RECIST), which may not be an optimal determinant of resistance to targeted agents (97). Targeted therapies can induce central necrosis, alter tumour vascularity, and retard tumour growth without reducing tumour size. Taking these changes into account, Choi criteria have been examined in the context of targeted therapy in RCC (98,99). In summary, switching to second-line treatment should be prompted by objective criteria along with clinical judgment. Sonpavde et al. propose a formal evaluation of continuing the same agent in patients with RECIST progression unaccompanied by symptoms (28).
Under the circumstances of true tumour progression despite adequate dosing, a central question is whether to maintain the therapeutic target or to change the mechanism of action of the antineoplastic drug, i.e., changing to another VEGFR-TKI or mTOR inhibitor, respectively.
Mechanisms of resistance to anti-angiogenic therapy and implications for further therapy
Bergers and Hanahan (100) have reviewed possible mechanisms of adaptive resistance to anti-angiogenic therapy. One of these mechanisms is up-regulation of alternative pro-angiogenic pathways. First clues for this hypothesis came from animal models in which higher mRNA expression levels for different pro-angiogenic factors were observed after blockage of VEGFR-signalling in pancreatic neuroendocrine cancer cells (80). Further studies showed up-regulation of pro-angiogenic factors such as PDGF and FGF (101) after angiogenesis inhibition. Moreover, the hypoxic environment caused by anti-VEGF therapy may lead to activation of the mTOR-pathway which integrates information about nutrients and growth factors and holds a central role in cell growth, cell cycle progression and coping with metabolic stress (102,103).
There is also growing evidence that the tumour microenvironment is crucial in adaptive resistance to anti-angiogenic therapy. For example, lower oxygen levels in tumours through VEGF-inhibition seem to lead to recruitment of vascular progenitor cells from the bone marrow. Experimentally induced ischaemia in tissues was shown to increase recruitment of bone marrow-derived cells and endothelial progenitors partly through elevated levels of HIF1 alpha (104,105). These progenitors may be able to maintain sufficient tumour angiogenesis even when VEGF-signalling is blocked.
Other studies could show that pericytes also seem to be of importance in acquiring resistance to anti-angiogenic therapy. Increased and thick coverage of vessels with these endothelial support cells was observed after VEGF-inhibition and may help to keep tumour vessels functioning (106,107).
Further investigations raise the hypothesis that cancer cells adapt to anti-angiogenic therapy by showing a more invasive phenotype and migrating more aggressively into normal tissues to ensure sufficient oxygen supply (108).
Other studies suggest an epithelial-to-mesenchymal transition (EMT) with acquisition of a sarcoma-like phenotype as a mechanism of escape from VEGF-inhibition. For example, Hammers et al. (109) described the case of a patient with initially pure clear cell RCC and response to sunitinib. After progression of the disease, a skin metastasis was excised and histologically showed EMT. After implantation into mice, clear cell histology as well as sensitivity to sunitinib was surprisingly restored. These observations underline importance of the tumour microenvironment for achieving resistance to anti-angiogenic therapy.
Taking into account all these possible mechanisms of acquiring resistance, certain considerations regarding optimal treatment sequence in metastatic and advanced RCC arise.
Activation of the mTOR pathway as a potential resistance principle creates the rationale for a change in therapeutic strategy after treatment with a first-line VEGFR-TKI. Blocking up-regulated mTOR signalling with an mTOR inhibitor such as everolimus or temsirolimus seems promising. Clinical proof of concept comes from the RECORD-1 trial, which showed significantly longer PFS for patients treated with everolimus in comparison to those on placebo after first-line treatment with a VEGFR-TKI (70).
Further arguments supporting a change of treatment principle occur considering the tumour microenvironment as described above. Epithelial-to-mesenchymal transition as a resistance mechanism to anti-VEGF therapy was reversed and sensitivity to sunitinib restored after excision and transplantation of a metastasis into mice (109). This observation argues for the concept of “drug holidays” to achieve a resetting of the original tumour microenvironment and re-establishing VEGF-dependency. Therefore, switching to a different therapeutic target in second-line therapy seems reasonable and may restore sensitivity to anti-VEGF therapy as a potential third-line option.
Observation of a more invasive tumour phenotype after anti-VEGF therapy further supports the concept of changing treatment mode (108,110). It should been taken into account that prolonged anti-angiogenic therapy may even be detrimental.
On the other hand, there is evidence arguing against a change of treatment principle. It is known from in vitro studies that treatment with mTOR inhibitors alone leads to tumour stimulating feedback mechanisms. mTOR contains two different complexes, the rapamycin-sensitive complex (Raptor, mTORC1) and the rapamycin-insensitive complex (Rictor, mTORC2). Available mTOR inhibitors for treatment of RCC such as everolimus und temsirolimus as well as the original macrolide rapamycin (sirolimus) only inhibit activation of the Raptor complex. It has been shown experimentally that inhibition of Raptor leads to increased stimulation of AKT/PKB due to Rictor (111,112). AKT/PKB is a protein kinase which implements a central role in regulation of cell growth and division, apoptosis and protein metabolism. This may even cause tumour growth and progression and limits the value of sole mTOR inhibition as a therapeutic principle. One further resistance mechanism has been proposed: a negative feedback loop activating the mitogen activated protein kinase (MAPK) signalling cascade, a separate oncogenic pathway (113). MAPK feedback activation was found to be PI3K-dependent (113).
In addition to mTOR, other pro-angiogenic factors such as PDGF and FGF have been shown to be up-regulated as a consequence of anti-angiogenic therapy. Therefore, patients progressing under anti-VEGF therapy may still show benefit from a VEGFR-TKI if the spectrum of inhibition is widened, for example by switching to a less-selective multi-kinase inhibitor such as sorafenib, which also inhibits PDGFR, c-KIT and Raf. This hypothesis is supported by results of the randomized phase III INTORSECT trial: sunitinib-resistant RCC patients were randomly assigned to treatment with either sorafenib or temsirolimus. Although no statistically significant difference in PFS could be observed, OS was longer for patients treated with sorafenib (75).
Interestingly, a third-line trial failed to show superiority of dovitinib, an inhibitor of both VEGFR and FGFR, over sorafenib in patients pre-treated with one anti-VEGF line and one line of an mTOR inhibitor (78). In her comment to this trial, M. Schmidinger raised the hypothesis that the timing of adding divotinib had been wrong rather than FGF as a target. Most patients in the trial (92%) had received a VEGF-inhibitor followed by an mTOR inhibitor. VEGF-inhibitor resistance has been suggested as being a temporary phenomenon due to changes of the tumour microenvironment. An “anti-VEGF drug holiday”, for example during mTOR inhibition, may restore dependency on VEGF-signalling and attenuate up-regulation of the FGF pathway. Therefore, it might have been more reasonable to analyse efficacy of a combined VEGF- and FGF-inhibitor directly after failure of VEGF-directed therapy than in the third line after additional failure of an mTOR inhibitor (114).
A further observation supporting maintenance of treatment with anti-VEGF therapeutics is the lack of complete cross-resistance regarding different anti-VEGFR TKIs (28). Results from the AXIS trial (73) showed that pre-treated patients of whom the majority received sunitinib as first-line treatment, demonstrated a significantly longer PFS when treated with axitinib in the second-line than with sorafenib. In terms of pharmacological activity, axitinib is a more potent VEGFR-inhibitor than sunitinib and sorafenib (IC50s 0.2 nM for axitinib, 80 nM for sunitinib and 90 nM for sorafenib). This creates a rationale for a treatment sequence weaker VEGFR TKI followed by stronger VEGFR TKI. Biologically, pre-treatment with a less potent drug of the same class may lead to a weaker selection pressure in tumour cells and therefore cause adaptive mechanisms which can still be overcome using a drug with greater inhibitory activity but the same spectrum of action.
Gerlinger et al. performed multiregion genetic analysis on spatially separated samples from primary RCC and associated metastatic sites using exome sequencing, chromosome aberration analysis, and ploidy profiling. Phylogenetic reconstruction revealed branched evolutionary tumour growth, with 63% to 69% of all somatic mutations not detectable across every tumour region. They found ubiquitous alterations in the trunk of the phylogenetic tree, such as allelic-imbalance events on chromosome 3p (encoding VHL), 5q, 6q, and 10q. However, heterogeneity was observed for a mutation within an auto-inhibitory domain of the mTOR kinase and for multiple tumour-suppressor genes converging on loss of function (115). The importance of targeting ubiquitous alterations in the trunk of the phylogenetic tree is underscored by branched tumour evolution. The difficulties encountered in the validation of oncology biomarkers owing to sampling bias may be explained by intratumour heterogeneity (116). In addition, this heterogeneity may contribute to Darwinian selection of preexisting drug-resistant clones (117,118) and predict resistance to treatments (119).
Taken together, there are arguments supporting both treatment strategies. Maintaining anti-VEGFR directed therapy as well as changing treatment principle in second-line seem reasonable and can be justified on a biological level (Figure 1). However, to date, no prospective data exist addressing the issue whether one strategy is superior to the other. In the end, clinical reasoning is still crucial in finding the best treatment strategy for an individual patient. Comorbidities and spectrum of adverse events have to be taken into account. Moreover, the individual biology of the disease, determining the degree of aggressiveness, seems to be the most important factor of all as demonstrated by two cases (Figures 2 and 3).
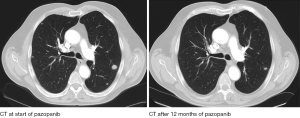
Considering different courses of presumably the same disease, there seems to be a divide between patients with slow progression and repeated treatment responses, and those with an aggressive phenotype, rapidly succumbing to their tumour (120).
As to the latter, there may be those with intrinsic, pre-existing non-responsiveness to anti-angiogenic therapy. Bergers and Hanahan (100) envision a tumour phenotype intrinsically expressing a plethora of pro-angiogenic factors and therefore being indifferent to anti-VEGFR therapy. This hypothesis is supported by an Italian retrospective study (77). In this analysis, patients did not show response to treatment with third-line sorafenib if there had already been lack of response to first-line sunitinib. This observation suggests existence of a primarily resistant phenotype concerning anti-VEGF therapy.
Furthermore, pre-existing inflammatory cell mediated vascular protection could be seen as another mechanism of intrinsic resistance to anti-angiogenic therapy. Animal studies could show pre-existing infiltration of inflammatory myeloid cells expressing pro-angiogenic factors in murine transplant tumours non-responsive to anti-VEGF directed therapy (104). The RECORD-1 trial (70) could also identify inflammation (elevated neutrophils) as an independent prognostic factor for shorter PFS and OS.
At the opposite end, we see patients showing long-term disease control with first-line anti-VEGF therapy. It is tempting to assume that those patients should best continue anti-VEGF directed therapy in the second-line. However, results from a European retrospective study (121) suggested that long-term first-line VEGFR-TKI responders may benefit from both, further VEGFR-TKI or mTOR inhibitors in the second-line. Taken together, for these patients the sequence of therapy may only be of minor relevance.
Conclusions and future perspective
There is increasing evidence of the central role of the VEGF/VEGFR-pathway in the development of RCC and good rationale for inhibition of this pathway due to the frequent mutation of the VHL tumour suppressor gene in ccRCC also in sporadic forms of the disease. This molecular hallmark renders RCC particularly dependent on angiogenesis and thus susceptible to angiogenesis inhibition with targeted agents (83). On the basis of this biologic understanding many new drugs have been developed for the treatment of RCC. In this review we present the clinical trials on targeted therapy in RCC. We point to the challenge in interpreting the data and in deriving the optimal treatment sequence. Trial design in RCC in the past was not only driven by scientific rationale but also by the interest of pharmaceutical companies to obtain marketing authorization. In fact, some drugs were used as a comparator in clinical setting not supported by previous evidence and not reflecting current daily practice.
Sequencing treatment is exclusively relevant to patients who are offered a second- or third-line treatment and who remain well enough to receive this treatment. Retrospective French data show that only 59% of patients received second-line treatment after sunitinib, 52% after sorafenib, and 79% after bevacizumab, respectively (122). Following first-line VEGF-targeted therapy 33% of 645 patients received second-line VEGF-targeted therapy or mTOR inhibiting agents (123). Similarly, 13% of patients received third-line treatment in an Italian retrospective analysis of targeted therapies (124). The data suggests that MSKCC risk groups and first-line therapy may be predictive factors for receiving second-line treatment. PFS was shown to be similar in the second- and third-line settings in a retrospective analysis of RECORD-1 patients (125). Hence, is the sequence relevant after all or is it merely a matter of favourable risk and access to drugs? And how important are toxicity management issues and correct assessment of disease progression?
The challenges are ahead. Novel immunotherapeutic agents have entered the field in RCC (126) and require integration in treatment algorithms and rethinking of the treatment sequence.
Acknowledgements
We thank Brian Meehan for critically reviewing grammar and style.
Footnote
Conflicts of Interest: S Fischer, None. S Gillessen: Bayer, Consultant Scientific Advisory Board and Speakers Bureau (uncompensated); Novartis, Consultant Scientific Advisory Board; Pfizer, Consultant Scientific Advisory Board. C Rothermundt: Pfizer, Consultant Scientific Advisory Board; GSK, Honoraria; Novartis, Honoraria.
References
- McKibbin T, Frei CR, Greene RE, et al. Disparities in the use of chemotherapy and monoclonal antibody therapy for elderly advanced colorectal cancer patients in the community oncology setting. Oncologist 2008;13:876-85. [PubMed]
- Gronlund B, Høgdall C, Christensen IJ, et al. Is stabilization of disease a useful indicator for survival in second-line treatment of ovarian carcinoma pre-treated with Paclitaxel-Platinum? Gynecol Oncol 2004;94:409-15. [PubMed]
- Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 2012;104:1534-41. [PubMed]
- Koopman M, Antonini NF, Douma J, et al. Randomised study of sequential versus combination chemotherapy with capecitabine, irinotecan and oxaliplatin in advanced colorectal cancer, an interim safety analysis. A Dutch Colorectal Cancer Group (DCCG) phase III study. Ann Oncol 2006;17:1523-8. [PubMed]
- Seymour MT, Maughan TS, Ledermann JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143-52. [PubMed]
- Dear RF, McGeechan K, Jenkins MC, et al. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2013;12:CD008792. [PubMed]
- Amadori D, Silvestrini R, De Lena M, et al. Randomized phase III trial of adjuvant epirubicin followed by cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) versus CMF followed by epirubicin in patients with node-negative or 1-3 node-positive rapidly proliferating breast cancer. Breast Cancer Res Treat 2011;125:775-84. [PubMed]
- Heinemann V, Vehling-Kaiser U, Waldschmidt D, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the ’Arbeitsgemeinschaft Internistische Onkologie’ (AIO-PK0104). Gut 2013;62:751-9. [PubMed]
- Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol 2013;71:829-42. [PubMed]
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [PubMed]
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [PubMed]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. [PubMed]
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33. [PubMed]
- Yap TA, Zivi A, Omlin A, et al. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:597-610. [PubMed]
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23:8253-61. [PubMed]
- Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309-22. [PubMed]
- Aggarwal R, Halabi S, Kelly WK, et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer 2013;119:3636-43. [PubMed]
- Meador CB, Jin H, de Stanchina E, et al. Optimizing the sequence of anti-EGFR-targeted therapy in EGFR-mutant lung cancer. Mol Cancer Ther 2015;14:542-52. [PubMed]
- Ahmad T, Eisen T. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res 2004;10:6388S-92S. [PubMed]
- Rini BI. New strategies in kidney cancer: therapeutic advances through understanding the molecular basis of response and resistance. Clin Cancer Res 2010;16:1348-54. [PubMed]
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii49-56. [PubMed]
- Bellmunt J, Puente J, Garcia de Muro J, et al. SEOM clinical guidelines for the treatment of renal cell carcinoma. Clin Transl Oncol 2014;16:1043-50. [PubMed]
- Ljungberg B, Bensalah K, Canfield S, et al. EAU Guidelines on Renal Cell Carcinoma: 2014 Update. Eur Urol 2015;67:913-24. [PubMed]
- Porta C, Szczylik C, Escudier B. Combination or sequencing strategies to improve the outcome of metastatic renal cell carcinoma patients: a critical review. Crit Rev Oncol Hematol 2012;82:323-37. [PubMed]
- Sonpavde G, Choueiri TK, Escudier B, et al. Sequencing of agents for metastatic renal cell carcinoma: can we customize therapy? Eur Urol 2012;61:307-16. [PubMed]
- Escudier B, Gore M. Sequencing therapy in metastatic renal cell cancer. Semin Oncol 2013;40:465-71. [PubMed]
- Schmidinger M. Improving outcomes in metastatic clear cell renal cell carcinoma by sequencing therapy. Am Soc Clin Oncol Educ Book 2014.e228-38. [PubMed]
- Albiges L, Choueiri T, Escudier B, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol 2015;67:100-10. [PubMed]
- Johannsen M, Staehler M, Ohlmann CH, et al. Outcome of treatment discontinuation in patients with metastatic renal cell carcinoma and no evidence of disease following targeted therapy with or without metastasectomy. Ann Oncol 2011;22:657-63. [PubMed]
- Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol 2012;30:482-7. [PubMed]
- Boxer RJ. A Conversation With Arie Belldegrun, MD, FACS, and Allan Pantuck, MD, MS, FACS. The ASCO Post 2014;5. Available online: http://www.ascopost.com/issues/april-15,-2014/a-conversation-with-arie-belldegrun,-md,-facs,-and-allan-pantuck-md,-ms,-facs.aspx
- Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:2765-72. [PubMed]
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289-96. [PubMed]
- McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:133-41. [PubMed]
- Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet 1999;353:14-7. [PubMed]
- Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunothérapie. N Engl J Med 1998;338:1272-8. [PubMed]
- Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007;110:2468-77. [PubMed]
- Geertsen PF, Gore ME, Negrier S, et al. Safety and efficacy of subcutaneous and continuous intravenous infusion rIL-2 in patients with metastatic renal cell carcinoma. Br J Cancer 2004;90:1156-62. [PubMed]
- Negrier S, Maral J, Drevon M, et al. Long-term follow-up of patients with metastatic renal cell carcinoma treated with intravenous recombinant interleukin-2 in Europe. Cancer J Sci Am 2000;6 Suppl 1:S93-8. [PubMed]
- Vogelzang NJ. Treatment options in metastatic renal carcinoma: an embarrassment of riches. J Clin Oncol 2006;24:1-3. [PubMed]
- Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol 2015;67:740-9. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [PubMed]
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103-11. [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40. [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [PubMed]
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [PubMed]
- Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422-8. [PubMed]
- Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137-43. [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [PubMed]
- Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287-96. [PubMed]
- Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol 2014;32:1412-8. [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [PubMed]
- Motzer RJ, Hutson TE, McCann L, et al. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769-70. [PubMed]
- Sun M, Trinh QD, Perrotte P, et al. Words of wisdom: Re: Pazopanib versus sunitinib in metastatic renal-cell carcinoma. Eur Urol 2014;65:1014-5. [PubMed]
- Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791-9. [PubMed]
- Garnick MB. Preserving the sanctity of overall survival for drugs approved on the basis of progression-free survival: tivozanib as a case study. J Clin Oncol 2013;31:3746-8. [PubMed]
- Harrison MR, George DJ, Walker MS, et al. “Real world” treatment of metastatic renal cell carcinoma in a joint community-academic cohort: progression-free survival over three lines of therapy. Clin Genitourin Cancer 2013;11:441-50. [PubMed]
- Fay AP, Xie WL, Lee JL, et al. Characteristics of long-term and short-term survivors of metastatic renal cell carcinoma treated with targeted therapies: results from the International mRCC Database Consortium. Clin Genitourin Cancer 2015;13:150-5. [PubMed]
- Ibrahim A, Arrington J, Jarrow J, et al. Tivozanib for the treatment of advanced renal cell cancer. Available online: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM351322.pdf
- Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:1280-9. [PubMed]
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287-94. [PubMed]
- Yousaf N, Larkin J. Axitinib in advanced renal-cell carcinoma. Lancet Oncol 2013;14:1245-6. [PubMed]
- Hanzly M, Aboumohamed A, Yarlagadda N, et al. High-dose interleukin-2 therapy for metastatic renal cell carcinoma: a contemporary experience. Urology 2014;83:1129-34. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009;27:3312-8. [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [PubMed]
- Calvo E, Escudier B, Motzer RJ, et al. Everolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer 2012;48:333-9. [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [PubMed]
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62. [PubMed]
- Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:760-7. [PubMed]
- Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 2008;26:3743-8. [PubMed]
- Di Lorenzo G, Buonerba C, Federico P, et al. Third-line sorafenib after sequential therapy with sunitinib and mTOR inhibitors in metastatic renal cell carcinoma. Eur Urol 2010;58:906-11. [PubMed]
- Motzer RJ, Porta C, Vogelzang NJ, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:286-96. [PubMed]
- Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer cell 2005;8:299-309. [PubMed]
- Grünwald V, Seidel C, Fenner M, et al. Treatment of everolimus-resistant metastatic renal cell carcinoma with VEGF-targeted therapies. Br J Cancer 2011;105:1635-9. [PubMed]
- Zama IN, Hutson TE, Elson P, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer 2010;116:5400-6. [PubMed]
- Grünwald V, Weikert S, Seidel C, et al. Efficacy of sunitinib re-exposure after failure of an mTOR inhibitor in patients with metastatic RCC. Onkologie 2011;34:310-4. [PubMed]
- Porta C, Paglino C, Grunwald V. Sunitinib re-challenge in advanced renal-cell carcinoma. Br J Cancer 2014;111:1047-53. [PubMed]
- Maj-Hes A, Medioni J, Scotte F, et al. Rechallenge with mTOR inhibitors in metastatic renal cell carcinoma patients who progressed on previous mTOR inhibitor therapy. Oncology 2013;85:8-13. [PubMed]
- Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol 2009;182:29-34; discussion 34. [PubMed]
- Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest 1990;86:670-4. [PubMed]
- de Paulsen N, Brychzy A, Fournier MC, et al. Role of transforming growth factor-alpha in von Hippel--Lindau (VHL)(-/-) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci U S A 2001;98:1387-92. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Pili R, Adelaiye R, Miles KM, et al. Overcoming sunitinib-induced resistance by dose escalation in renal cell carcinoma: Evidence in animal models and patients. J Clin Oncol 2013;31:abstr 4582.
- Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010;66:357-71. [PubMed]
- Amato R, Zhai J, Willis J, et al. A phase II trial of intrapatient dose-escalated sorafenib in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2012;10:153-8. [PubMed]
- Mancuso A, Di Paola ED, Leone A, et al. Phase II escalation study of sorafenib in patients with metastatic renal cell carcinoma who have been previously treated with anti-angiogenic treatment. BJU Int 2012;109:200-6. [PubMed]
- Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol 2013;53:491-504. [PubMed]
- Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol 2013;14:1233-42. [PubMed]
- Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 2002;94:652-61. [PubMed]
- Barthélémy P, Asmane-De la Porte I, Meyer N, et al. Adherence and patients' attitudes to oral anticancer drugs: a prospective series of 201 patients focusing on targeted therapies. Oncology 2015;88:1-8. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 2010;102:803-9. [PubMed]
- Schmidt N, Hess V, Zumbrunn T, et al. Choi response criteria for prediction of survival in patients with metastatic renal cell carcinoma treated with anti-angiogenic therapies. Eur Radiol 2013;23:632-9. [PubMed]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008;8:592-603. [PubMed]
- Fernando NT, Koch M, Rothrock C, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res 2008;14:1529-39. [PubMed]
- Edinger AL, Thompson CB. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene 2004;23:5654-63. [PubMed]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004;23:3151-71. [PubMed]
- Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007;25:911-20. [PubMed]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858-64. [PubMed]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005;7:452-64. [PubMed]
- Jain RK, Booth MF. What brings pericytes to tumor vessels? J Clin Invest 2003;112:1134-6. [PubMed]
- Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2000;2:306-14. [PubMed]
- Hammers HJ, Verheul HM, Salumbides B, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther 2010;9:1525-35. [PubMed]
- Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009;15:220-31. [PubMed]
- Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004;14:1296-302. [PubMed]
- Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 2005;65:7052-8. [PubMed]
- Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 2008;118:3065-74. [PubMed]
- Schmidinger M. Third-line dovitinib in metastatic renal cell carcinoma. Lancet Oncol 2014;15:245-6. [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [PubMed]
- Poste G. Bring on the biomarkers. Nature 2011;469:156-7. [PubMed]
- Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res 2006;66:7854-8. [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [PubMed]
- Lee AJ, Endesfelder D, Rowan AJ, et al. Chromosomal instability confers intrinsic multidrug resistance. Cancer Res 2011;71:1858-70. [PubMed]
- Heng DY, Mackenzie MJ, Vaishampayan UN, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol 2012;23:1549-55. [PubMed]
- Elaidi RT, Beuselinck B, Maj-Hes A, et al. What is the best treatment option for second line in long responders to the first-line TKI in metastatic renal cell carcinoma (mRCC) patients (pts): TKI-TKI or TKI-mTORi- A European retrospective study. J Clin Oncol 2012;30:abstr 375.
- Levy A, Menard J, Albiges L, et al. Second line treatment of metastatic renal cell carcinoma: The Institut Gustave Roussy experience with targeted therapies in 251 consecutive patients. Eur J Cancer 2013;49:1898-904. [PubMed]
- Vickers MM, Choueiri TK, Rogers M, et al. Clinical outcome in metastatic renal cell carcinoma patients after failure of initial vascular endothelial growth factor-targeted therapy. Urology 2010;76:430-4. [PubMed]
- Iacovelli R, Cartenì G, Sternberg CN, et al. Clinical outcomes in patients receiving three lines of targeted therapy for metastatic renal cell carcinoma: results from a large patient cohort. Eur J Cancer 2013;49:2134-42. [PubMed]
- Blesius A, Beuselinck B, Chevreau C, et al. Are tyrosine kinase inhibitors still active in patients with metastatic renal cell carcinoma previously treated with a tyrosine kinase inhibitor and everolimus? Experience of 36 patients treated in France in the RECORD-1 Trial. Clin Genitourin Cancer 2013;11:128-33. [PubMed]
- Bailey A, McDermott DF. Immune checkpoint inhibitors as novel targets for renal cell carcinoma therapeutics. Cancer J 2013;19:348-52. [PubMed]


