
Sexual function in the penile cancer survivor: a narrative review
Introduction
Squamous cell carcinoma of the penis is a rare malignancy among men in North America and Europe with an incidence of <1 per 100,00 men, but may represent an increasing percentage of neoplasms affecting men in South American, African, and Asian countries (1,2). The considerable worldwide variation in geographical incidence is unclear, but may be centered around the etiologic risk factors of this neoplasm including socioeconomic factors as well as hygienic and cultural habits (1,3). Penile carcinoma can affect older, often still sexually active men, with 55% of penile cancer patients being 60 years of age or younger at the time of diagnosis (4). Squamous cell carcinoma is the predominant histologic type with the primary lesion involving the glans in 48% of cases, prepuce in 21%, both glans and prepuce in 9%, coronal sulcus in 6% and less than 2% involving the shaft of the penis (5). The treatment modalities of penile carcinoma span the gamut from organ-sparing treatments such as topical therapy, laser therapy, radiotherapy, glansectomy, wide-local excision and partial penectomy. Those with more advanced primary disease may require more genital mutilating procedures such as total penectomy.
All treatments can have the potential to provide disfigurement and subsequently impact quality of life (QOL) parameters, including sexual function (6). Of all genitourinary cancers, penile carcinoma has the potential to jeopardize sexual function the most, with a large number of patients reporting anxiety at the prospect of interference with sex life (7). Quality of life is an important endpoint in the cancer-care arena, and well-documented data derived from penile cancer survivors is lacking in the medical literature. As more patients achieve longer-term survival after treatment of penile cancer, sexual dysfunction is an increasingly recognized consequence affecting quality of life (8). Despite the possibility of local recurrence which varies based on treatment modality, 5-year-disease-specific survival exceeds 90%, and these patients are therefore likely to bear the sexual and psychosexual effects of penile cancer treatment for a long time after diagnosis and treatment (2). Knowledge of how this disease process affects sexual outcomes in the penile cancer survivor better equips the physician with the resources and education to properly counsel the patient with penile carcinoma.
Due to the rarity of this disease, there is a relative paucity of data in the medical literature describing the impact of penile cancer treatment on sexual function. Table 1 summarizes the main available data in the medical literature evaluating sexual function outcomes in penile cancer patients. Quality of life after penile cancer treatment, particularly in the arena of sexual function, is an important point of discussion between the treating physician and the patient. Herein, the purpose of this review article is to examine the sexual function outcomes of patients who undergo various treatment modalities for carcinoma of the penis.
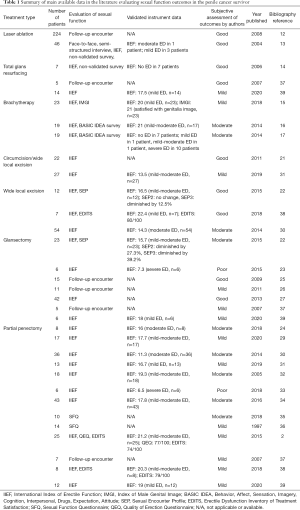
Full table
Penectomy is traditionally considered the oncologic gold-standard for definitive treatment of the primary penile tumor, the mainstay of which relies upon wide excision of the lesion by partial or total penectomy approach (9). While surgical amputation of the primary tumor results in good oncological outcomes, this approach has been shown to have significant impact on sexual quality of life. More recently; however, a variety of organ-sparing techniques have been described to preserve as much penile tissue and functional integrity as possible to mitigate the impact on sexual function outcomes without compromising oncologic control (9,10). The purpose of this review article is not to describe the limitations or indications of penile-sparing procedures, but to examine their respective impact on sexual function in the penile cancer survivor. We present the following article in accordance with the Narrative Review Checklist (available at: http://dx.doi.org/10.21037/tau-20-1228).
Laser ablation
In their review of literature, Audenet et al. reported on sexual function outcomes and satisfaction after conservative treatment of penile carcinoma with laser ablation (11). In one series of patients who underwent CO2 laser excision in 224 patients reported no functional impairments or changes in erection capacity following this type of treatment (12). In a retrospective, face-to-face, semi-structured interview, Windahl et al. investigated sexual function and satisfaction after laser treatment in 46 Swedish men using a 53-item questionnaire (13). Forty patients were sexually active prior to laser treatment, and only 30 men had resumed sexual activity post-operatively; only 50% (n=23) of the cohort was either satisfied or very satisfied with their sex life, and 72% (n=33) considered their sex life to be as good as they had wanted (13). The study reported that this cohort of patients had largely the same level of satisfaction with sex life as those in the general Swedish population of all men aged 18 to 74 years old. Twenty-two percent (n=10) of the study participants reported a decrease in erectile function, 72% reported unchanged erectile function, and 6% reported improved function after laser treatment. Only the 10 patients who reported decreased erectile function were asked to complete the International Index of Erectile Function questionnaire (IIEF-5). Three reported mild erectile dysfunction (ED), 1 reported moderate ED, the rest (n=6) reported no sexual activity. Dyspareunia was experienced by 7 patients prior to treatment and caused distress for 6, which eventually resolved in all but one patient. In total, dyspareunia after treatment was distressing for 3 patients. The treating physician should be aware of the risk of dyspareunia as this may cause distress, and to some degree, decreased sexual interest (13). Together, these studies demonstrate that sexual outcomes are largely preserved after laser treatment of penile carcinoma but are limited in that neither study fully utilized a validated survey instrument to quantify or characterize sexual outcomes and are largely reported based upon individual patient encounters.
An alternative primary treatment to laser ablation for superficial noninvasive disease is total glans resurfacing. A study from England reported sexual function outcomes in 7 patients who were treated with total glans resurfacing. All patients were able to return to regular sexual activity within 3–5 months after undergoing treatment. The median IIEF-5 score in this cohort of 7 men was 24, corresponding to no erectile dysfunction. A 9-item, non-validated questionnaire was also administered. There were no reported changes in glans sensation, and all 7 men had the ability to obtain erections within 2–3 weeks after total glans resurfacing. The authors reported an overall high rate of patient satisfaction with glans resurfacing in that 5 patients (71%) reported that their sex life had in fact improved; the rest reported no change (14).
Brachytherapy
Brachytherapy is an organ-preserving treatment modality for patients with localized penile carcinoma. Gambachidze et al. in their retrospective single-center study published their findings on the impact of brachytherapy on sexual and quality of life outcomes between 1991 and 2015 (15). There were 23 eligible study participants who were administered the validated IIEF-5 and EuroQol questionnaires to assess sexual and quality of life outcomes, respectively. The impact of brachytherapy on sexual activity was moderate—70.8% of patients maintained sexual intercourse with a median IIEF-5 score of 20, corresponding to mild ED. Despite good functional outcomes, nine patients complained of moderate pain post-operatively. The authors also implemented the Index of Male Genitalia Image (IMGI) tool to assess patient perceptions of their male genitalia image; the median score was 21, indicating that patients in this cohort were generally satisfied with the image of their genitalia.
To elaborate on the impact of brachytherapy on sexual outcomes, Soh et al. matched 19 men treated with penile brachytherapy and 19 age-matched controls who participated in a first of its kind study comparing sexual function and psychosexual QOL (16). Eighty-nine percent of men were sexually active prior to treatment, though only 36.8% had frequent intercourse. After treatment, the proportion of sexually inactive men increased from 10.5% to 47.3%. Men experiencing ED increased from 21.1% to 57.8%, and 42% of men reported new anorgasmia and anejaculation. All parameters of sexual functioning such as frequency of sexual intercourse, erectile dysfunction, ejaculation, orgasm, and nocturnal erections exhibited a statistically significant decrease between pre- and post-treatment brachytherapy. Despite these findings, 11 patients (57.9%) reported being very satisfied with sex life and 8 (42.1%) reported average satisfaction. No patient in this study reported dissatisfaction with sex life after treatment. Interestingly, when comparing the IIEF scores between the two cohorts, the erectile function, sexual desire, and overall satisfaction domain scores were in fact statistically significantly higher in the treatment group than the control group who otherwise had no evidence of penile pathology. The IIEF-5 score of the treatment group and control group corresponded to mild to moderate ED and moderate ED, respectively. The major limitation of this study is highlighted in that 63% of the controls never had sexual intercourse and 74% reported rarely or never having experienced erections, explaining the lower IIEF scores.
Delaunay and colleagues, in their cohort of 19 men described that 59% of patients who were sexually active prior to penile brachytherapy remained as such after treatment, and 94% who experienced erections prior to treatment had maintenance of their erections post-brachytherapy (17). Using the IIEF-5, the authors reported that 52.6% (n=10) had severe erectile dysfunction and 36.8% (n=7) had no erectile dysfunction after treatment. The authors also found that 68% (n=13) were either rarely or never participating in sexual intercourse post-operatively despite having erections often or sometimes. The rest (n=6) were only often or sometimes participating in sexual intercourse. Ten patients had hard or almost-hard erections and the rest (n=7) described either moderately hard or soft erections. In general, 58% of men were very satisfied with sex life and none were dissatisfied. In one of the earliest studies of its kind, Opjordsmoen and colleagues in 1994 demonstrated intact social adjustment and good psychological outcomes in those treated with surgery and radiotherapy (18,19). Sexual function impairment was greater in those who were treated with penectomy than their counterparts who received radiotherapy (20). The data remains mixed, however. Prior to these studies, information regarding sexual outcomes in patients treated with penile brachytherapy was scarce. Although several additional studies report on persistence of sexual function after penile brachytherapy treatment, many do not implement data gathered from validated instruments or control groups.
Wide local excision
To evaluate sexual outcomes of organ-sparing surgery in urban Shanghai, Li and colleagues enrolled 29 patients who were treated with circumcision, wide local excision (WLE), or both (21). Patients were administered the IIEF-5 preoperatively and again at 3 months postoperatively. Preoperative baseline sexual function was reported as moderate to severe erectile dysfunction in 7 (24.1%) and none to mild erectile dysfunction in 22 patients (75.9%). At the 3-month postoperative visit, only 1 (4.5%) patient had mild-moderate ED and the rest of the respondents (n=21) returned to their baseline degree of sexual function. Unsurprisingly, these findings suggest that organ-sparing treatment with radical circumcision with or without concomitant WLE appears to preserve sexual function in the penile cancer patient.
Wide local excision with or without glans reconstruction is an organ-sparing alternative which is oncologically suitable yet still confers the benefits of a maximally conservative procedure (22). Sedigh et al. retrospectively reviewed and compared the sexual outcomes in 12 patients who underwent WLE and 23 patients who underwent glansectomy with urethral glanduloplasty. The authors found that WLE achieved the best outcomes in terms of sexual function. WLE did not affect erectile function or future penetrative intercourse in this cohort of patients. These findings were confirmed by administering the IIEF-15 and Sex Encounter Profile (SEP) questionnaires both pre- and post-operatively. Erectile function, orgasmic function and intercourse satisfaction domain scores remained the same for those treated with WLE, whereas the sexual desire and overall satisfaction domain scores decreased. Those treated with glansectomy with urethral glanduloplasty exhibited lower scores in the erectile function, orgasmic function, sexual desire, and overall satisfaction domain scores. As for the SEP, while no significant changes were noted in the WLE group, a notable reduction was reported for the latter group with a decrease in the possibility of achieving penetrative intercourse and perceived satisfaction, both of which were statistically significant. The authors reported that WLE did not affect erectile function or future penetrative intercourse, but that glansectomy not only affected both domains, it affected erectile function, orgasmic function, and overall satisfaction domains the most. The drawback to glansectomy from a sexual function standpoint is apparent in that it can lead to penile shortening and reduce glanular sensation during intercourse. Although some sensation can be regained after glans reconstruction, this is not comparable to preoperative sensation (22).
Glansectomy
Six patients were treated with organ-sparing surgery for penile carcinoma at the Cleveland Clinic, 4 of whom underwent distal corporectomy and 2 underwent glansectomy only (23). Using the validated IIEF-15, Scarberry et al. demonstrated that patients who underwent glansectomy and/or distal corporectomy reported poor erectile function post-operatively. Half of the cohort had poor erectile quality and 67% reported no sexual activity despite all having been sexually active prior to treatment. Notably, despite poor sexual function outcomes, all patients reported satisfaction with the procedure outcome and reported quality of life, which was similarly reported by Park et al. (24). These findings differ from an European study, where Morelli et al. published favorable functional outcomes in 15 patients who underwent total glans amputation with neoglans reconstruction (25). All 15 patients had maintenance of sexual activity at 3 months after the surgery with preserved orgasm and ejaculation function. The ability to perform vaginal penetration remained intact, suggestive that sexual function in this cohort of men undergoing glansectomy with reconstruction was generally preserved. All patients admitted to reduced sensitivity in the neoglans as a predictable consequence of glans amputation but were able to maintain orgasm due to presence of erogenous sensation from the tip of the corpus cavernosum (25). O’Kane et al. also reported that 82% (n=9) of their cohort was able to achieve erections and 54% (n=6) remained sexually active after glansectomy (26). Notably however, unlike the previous study by Scarberry et al., the studies by Morelli et al. and O’Kane et al. did not use validated instruments to assess sexual function. The conclusions are limited by a small patient sample and a lack of control group limiting statistical analysis. Encouraging findings are reported by Gulino et al., who assessed sexual function using the IIEF-15 3 months before glandulectomy with glanduloplasty and 6 months postoperatively in 42 patients. There was no statistically significant change in sexual function based on IIEF-15 scores, demonstrating recovery of rigid erections and sexual function, along with preserved libido and ejaculation function (27).
Partial penectomy
Due to the rarity of penile cancer, there are few available studies that explore sexual outcomes after penile extirpative surgery. Radical penile surgery achieves excellent local control rates and is considered the oncologic gold standard; however, it can be associated with significant psychological morbidity and sexual dysfunction (10,18,19). Radical partial or total penectomy has been described to affect many quality of life metrics, including psychosexual parameters such as confidence, male self-image, and sexual function. In one of the largest retrospective reviews of North American penile cancer patients, Park et al. identified 34 cases of penile cancer who were treated with partial or total penectomy over a 10-year period at a single center institution (24). Eleven met the inclusion criteria; 8 underwent partial penectomy and 3 underwent total penectomy. Postoperatively, 6 patients were satisfied with their sex life, 2 reported equivocal satisfaction, and 3 were dissatisfied. Interestingly, 80% of the entire patient cohort reported satisfaction with the outcome of their operation. The authors compared the cohort’s postoperative IIEF-15 scores to a series of 109 healthy males without documented erectile dysfunction. Regarding sexual domain scores, the study cohort scored only 37% lower on erectile function, 44% lower on orgasmic function, 38% lower on intercourse satisfaction, and 32% lower on overall satisfaction (24,28). The 3 patients who were treated with total penectomy did report some degree of sexual satisfaction despite the inability to have penetrative intercourse, suggesting modalities other than intercourse may confer some degree of sexual satisfaction in this patient population (24). In a 5-year follow-up study to the original, Stroie et al. report encouraging preliminary outcomes in their cohort of 17 sexually active patients treated with partial penectomy over a 16-year period, making it the largest series of its kind studying an American population (29). Of the 17 patients who underwent partial penectomy, the mean IIEF-15 erectile function domain score was 17.7 corresponding to mild-moderate ED. The median orgasmic function was 6/10, the median intercourse satisfaction was 9/15 and the median overall sexual satisfaction was 7.5/10. Accounting for the entire cohort, 70% of patients reported moderate satisfaction or greater in overall satisfaction with sex life, and 90% of patients reported overall satisfaction with the outcome of their operation (29).
In a similar study, Kieffer et al. assessed sexual function with the IIEF-15 in a pool of 90 men treated for penile cancer with laser/local excision with or without circumcision, glansectomy with or without reconstruction, and partial penectomy (30). Similar to Park and colleagues, the authors compared the IIEF-15 domain scores to that of 109 healthy males without erectile dysfunction. The heterogeneous cohort scored 50% lower in the erectile function, orgasmic function, and intercourse satisfaction domains, 2% lower in the sexual desire domain, and 41% lower in the overall satisfaction domain of the IIEF. The authors found that those treated with organ-preserving conservative treatment modalities exhibited statistically significant higher orgasmic function domain scores than their counterparts who underwent partial penectomy. Notably, no statistically significant difference was noted in the domains of erectile function, sexual desire, overall sexual satisfaction, or intercourse satisfaction; however, those treated with partial penectomy reported a higher degree of appearance concerns than their counterparts. A Polish study by Sosnowski et al. reported similar outcomes (31). Using the IIEF, the authors assessed sexual outcomes in 40 men using validated questionnaires in sexually active patients who underwent circumcision/WLE and partial penectomy. Those treated with circumcision/WLE or partial penectomy reported mild-moderate ED and mild ED, respectively. The differences were not statistically significant indicating that the type of procedure is not a significant predictor of post-treatment sexual outcomes.
To date, there is a relative paucity of data exploring sexual outcomes in patients treated with partial penectomy. The data is often heterogeneous and the use of validated instruments to quantify sexual outcomes is not widely implemented. As such, the overall disparity of results may reflect varying societal norms and differing methodology of each study. Romero et al. in his cohort of 18 men reported a statistically significant reduction in erectile and orgasmic function, sexual desire, intercourse satisfaction, and overall sexual satisfaction after partial penectomy (32).Using the IIEF-15, the study reported that 55.6% of men maintained erectile function suitable for intercourse and 72% maintained feelings of orgasm and ejaculation, though only a third of the cohort maintained preoperative levels of intercourse frequency. Those who did not return to baseline levels of sexual intercourse were attributed to loss of glanular sensation and reduction of penile size leading to feelings of shame and an altered masculine self-image (32). While limited by a small sample size, the study demonstrates an overall reduction in sexual outcomes for those who undergo partial penectomy, some of which is in part due to psychological factors. Similar findings were published by Suarez-Ibarrola et al. reporting severely affected sexual functioning in 6 men treated with partial penectomy, with an IIEF-5 score corresponding to severe erectile dysfunction (33). In a Chinese prospective study, Yu et al. demonstrated a statistically significant decrease in all postoperative IIEF-15 domain scores in 43 men who were treated with partial penectomy (34). Seven patients reported satisfaction, 28 reported equivocal satisfaction, and 8 were very dissatisfied with their overall sex life after surgery. Age and body mass index at time of partial penectomy was negatively associated with overall sexual satisfaction, whereas having a partner was positively associated with overall satisfaction. Additionally, rates of anxiety and depression were noted to be significantly increased after partial penectomy (34).
Bhat et al. described twelve patients, two had undergone total penectomy and ten underwent partial penectomy (35).The partial penectomy cohort had intact masculine self-image prior to treatment that was lost in all ten patients postoperatively. Interestingly, one patient treated with total penectomy did experience an orgasm-like sensation from stimulation of the scar at the penectomy root. Unsurprisingly, the patients who underwent total penectomy reported higher levels of sexual desire but less sexual satisfaction than their partial penectomy counterparts which affected the relationship with their partners. The authors reported an absolute overall reduction in sexual satisfaction among those treated with partial penectomy (35). The outcomes were poorer still in those treated with total penectomy.
Although a myriad of functional and psychosexual issues can be encountered with penectomy, there is encouraging data in the medical literature describing sexual outcomes. In one such study, D’Ancona et al. investigated 14 patients who underwent partial penectomy (36). The authors described favorable results, where the overall sexual function was normal or slightly decreased in 64% of patients. Fourteen percent had inconsistent or no sexual function. The relationship with their partners was unchanged in all patients despite undergoing genital mutilating surgery. Sexual interest and satisfaction was normal, or minimally reduced in 9 and 12 patients, respectively (36). All patients were sexually active with normal erectile function preoperatively, though 3 men did not resume sexual activity after penectomy. The authors reported that although partial penectomy confers a reduced penile length, vaginal penetration can still be achieved, and the ability to reach orgasm and ejaculation remains intact. This finding held true in the study by Park et al. and Stroie et al., where at least half of their cohort experienced preserved orgasmic function with not only satisfactory, but also enjoyable sexual intercourse. Furthermore, the authors state that in cases of partial penectomy, when the patient has the support of his partner, psychosocial and sexual quality of life can be maintained to levels similar to baseline function (36).
Sansalone et al. retrospectively enrolled 25 Italian patients who were treated with partial penectomy followed by pseudoglans reconstruction over a 3-year period (2).The authors implemented four standardized and validated questionnaires for data collection. The Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS), Quality of Erection Questionnaire (QEQ), Self-Esteem and Relationship Questionnaire (SEAR), and IIEF-15 questionnaires were used to assess patient satisfaction with erectile function after partial penectomy with glans reconstruction. Much of the cohort reported high degrees of confidence in achieving erections and 64% were able to achieve orgasm. The majority of the cohort also reported a decrease in the frequency of sexual intercourse for reasons of shame and embarrassment due to the remaining penile stump (2). A statistically significant difference was noted in all five IIEF-15 domains before and after surgery. Interestingly, the results of the SEAR and QEQ indicated that self-esteem and relationship satisfaction was generally maintained. Seventy-two percent of patients were confident in their ability to obtain an erection postoperatively and were generally highly satisfied with the outcome of their operation (2). Furthermore, Palminteri et al. in their series performed 5 total glans resurfacing procedures, 5 glansectomies with neoglans reconstruction, and 7 partial penectomies with neoglans reconstruction (37). Although no validated instrument was used, the authors reported that the neoglans cosmesis was similar to the true glans, and all patients were able to regain sexual activity in full. In fact, the aesthetic appearance of the penis was subjectively superior when compared to patients who underwent other organ-sparing techniques (37).
Medical literature exploring sexual outcomes between different surgical treatment modalities remains scarce. One such study by Wan et al. comparatively evaluated sexual function between patients who underwent partial penectomy and wide local excision (38). Sexual function was assessed by the IIEF-15 questionnaire pre- and post-operatively. Patients who underwent WLE had improved sexual outcomes after the fact, whereas the partial penectomy cohort exhibited mild impairment in sexual functioning in all 5 domains of the IIEF. Postoperative IIEF domain scores were higher in the WLE cohort than the partial penectomy counterparts; however, this was not found to be statistically significant except for the orgasmic function domain. The EDITS questionnaire in the demonstrated patients were largely satisfied with the outcome of their surgery, as were the patient’s partners. Opjordsmoen and colleagues report that organ-sparing surgical techniques, such as wide local excision and partial penectomy could lead to fewer impairments and similar sexual outcomes (19). This is demonstrated by a Colombian study where 14 patients underwent glans resurfacing, 6 underwent glansectomy, and 12 underwent partial penectomy (39). All were sexually active at time of study participation. The mean IIEF-5 scores corresponded to mild ED across all 3 cohorts, and there was no statistically significant difference when comparing sexual outcomes in any of the surgical groups. Interestingly, Opjordsmoen and colleagues reported that when asked about the treatment method of choice, a third of penile cancer patients would opt for the treatment option that confers a potentially poorer long-term survival to increase the chance of maintaining preserved sexual potency (18,19).
Conclusions
Although an uncommon genitourinary malignancy, a diagnosis of penile carcinoma carries implications for sexual function outcomes in those afflicted. Opportunities to study sexual outcomes in the penile cancer survivor are limited due to the lack of standardized clinical data. Additionally, the majority of available studies use retrospective data from small samples utilizing heterogeneous study tools such as patient interviews and non-validated questionnaires. A lack of consistency in the type of survey instrument used along with variability in data collection limits the ability to directly compare sexual outcomes. The most commonly used validated instrument to evaluate sexual outcomes is the International Index of Erectile Function Questionnaire (IIEF). The tool is limited in that it only analyzes those who are sexually active. Additionally, it does not assess a subset of patients who perform self-stimulation or achieve sexual stimulation by any means other than penetrative intercourse. Large, well-designed studies using validated instruments are needed in this arena to better assist the treating physician in navigating their patient’s sexual outcomes. The sexual outcomes after penile cancer treatment strategies were reviewed from the available published data to better assist the patient and the treating physician with medical decision making. With a detailed assessment of sexual outcomes, the physician is better equipped in providing patient centered care to achieve outcomes meaningful for each patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology for the series “Controversies and Considerations of Penile Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review Checklist. Available at: http://dx.doi.org/10.21037/tau-20-1228
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1459). The series “Controversies and Considerations of Penile Surgery” was commissioned by the editorial office without any funding or sponsorship. TSK served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Translational Andrology and Urology from Jan 2020 to Dec 2021. TSK reports consultant from Coloplast, consultant from Boston Scientific, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol 2007;25:361-7. [Crossref] [PubMed]
- Sansalone S, Silvani M, Leonardi R, et al. Sexual outcomes after partial penectomy for penile cancer: results from a multi-institutional study. Asian J Androl 2017;19:57-61. [PubMed]
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int 2013;112:21-6. [Crossref] [PubMed]
- Pettaway CA, Crook JM, Pagliaro LC. Tumors of the Penis. In: Wein AJ, Kavoussi LR, Partin AW et al., editors. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier, 2016:846-7.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005;96:1040-3. [Crossref] [PubMed]
- Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol 2009;9:8. [Crossref] [PubMed]
- Schover LR, von Eschenbach AC, Smith DB, et al. Sexual rehabilitation of urologic cancer patients: a practical approach. CA Cancer J Clin 1984;34:66-74. [Crossref] [PubMed]
- Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol 2010;57:1002-12. [Crossref] [PubMed]
- Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol 2016;69:377-83. [PubMed]
- Hegarty PK, Shabbir M, Hughes B, et al. Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: recommendations from the United Kingdom experience. World J Urol 2009;27:179-87. [Crossref] [PubMed]
- Audenet F, Sfakianos JP. Psychosocial impact of penile carcinoma. Transl Androl Urol 2017;6:874-8. [Crossref] [PubMed]
- Bandieramonte G, Colecchia M, Mariani L, et al. Peniscopically controlled CO2 laser excision for conservative treatment of in situ and T1 penile carcinoma: report on 224 patients. Eur Urol 2008;54:875-82. [Crossref] [PubMed]
- Windahl T, Skeppner E, Andersson SO, et al. Sexual function and satisfaction in men after laser treatment for penile carcinoma. J Urol 2004;172:648-51. [Crossref] [PubMed]
- Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int 2006;98:532-6. [Crossref] [PubMed]
- Gambachidze D, Lebacle C, Maroun P, et al. Long-term evaluation of urinary, sexual, and quality of life outcomes after brachytherapy for penile carcinoma. Brachytherapy 2018;17:221-6. [Crossref] [PubMed]
- Soh PN, Delaunay B, Nasr EB, et al. Evaluation of sexual functions and sexual behaviors after penile brachytherapy in men treated for penile carcinoma. Basic Clin Androl 2014;24:13. [Crossref] [PubMed]
- Delaunay B, Soh PN, Delannes M, et al. Brachytherapy for penile cancer: efficacy and impact on sexual function. Brachytherapy 2014;13:380-7. [Crossref] [PubMed]
- Opjordsmoen S, Fossa SD. Quality of life in patients treated for penile cancer. A follow-up study. Br J Urol 1994;74:652-7. [Crossref] [PubMed]
- Opjordsmoen S, Waehre H, Aass N, et al. Sexuality in patients treated for penile cancer: patients' experience and doctors' judgement. Br J Urol 1994;73:554-60. [Crossref] [PubMed]
- Ficarra V, Righetti R, D'Amico A, et al. General state of health and psychological well-being in patients after surgery for urological malignant neoplasms. Urol Int 2000;65:130-4. [Crossref] [PubMed]
- Li J, Zhu Y, Zhang SL, et al. Organ-sparing surgery for penile cancer: complications and outcomes. Urology 2011;78:1121-4. [Crossref] [PubMed]
- Sedigh O, Falcone M, Ceruti C, et al. Sexual function after surgical treatment for penile cancer: Which organ-sparing approach gives the best results? Can Urol Assoc J 2015;9:E423-7. [Crossref] [PubMed]
- Scarberry K, Angermeier KW, Montague D, et al. Outcomes for Organ-Preserving Surgery for Penile Cancer. Sex Med 2015;3:62-6. [Crossref] [PubMed]
- Park EJ, Stroie FA, Psutka SP, et al. Sexual and Voiding Outcomes in Post-Penectomy Penile Cancer Patients. JOJ uro & nephron 2018;6.
- Morelli G, Pagni R, Mariani C, et al. Glansectomy with split-thickness skin graft for the treatment of penile carcinoma. Int J Impot Res 2009;21:311-4. [Crossref] [PubMed]
- O'Kane HF, Pahuja A, Ho KJ, et al. Outcome of glansectomy and skin grafting in the management of penile cancer. Adv Urol 2011;2011:240824 [Crossref] [PubMed]
- Gulino G, Sasso F, Palermo G, et al. Sexual outcomes after organ potency-sparing surgery and glans reconstruction in patients with penile carcinoma. Indian J Urol 2013;29:119-23. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002;14:226-44. [Crossref] [PubMed]
- Stroie F, Fakhoury M, Houlihan M, et al. No, Your Sex Life is Not Over!: Long-term Functional Outcomes Following Penectomy. J Urol 2020;203:e137 [Crossref]
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol 2014;192:1105-10. [Crossref] [PubMed]
- Sosnowski R, Wolski JK, Zi Talewicz U, et al. Assessment of selected quality of life domains in patients who have undergone conservative or radical surgical treatment for penile cancer: an observational study. Sex Health 2019;16:32-8. [Crossref] [PubMed]
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology 2005;66:1292-5. [Crossref] [PubMed]
- Suarez-Ibarrola R, Cortes-Telles A, Miernik A. Health-Related Quality of Life and Sexual Function in Patients Treated for Penile Cancer. Urol Int 2018;101:351-7. [Crossref] [PubMed]
- Yu C, Hequn C, Longfei L, et al. Sexual Function after Partial Penectomy: A Prospectively Study From China. Sci Rep 2016;6:21862. [Crossref] [PubMed]
- Bhat GS, Nelivigi G, Barude V, et al. Sexuality in Surgically Treated Carcinoma Penis Patients and Their Partners. Indian J Surg 2018;80:19-23. [Crossref] [PubMed]
- D'Ancona CA, Botega NJ, De Moraes C, et al. Quality of life after partial penectomy for penile carcinoma. Urology 1997;50:593-6. [Crossref] [PubMed]
- Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and reconstruction of the glans penis. Eur Urol 2007;52:893-8. [Crossref] [PubMed]
- Wan X, Zheng D, Liu C, et al. A Comparative study of two types of organ-sparing surgeries for early stage penile cancer: Wide local excision vs partial penectomy. Eur J Surg Oncol 2018;44:1425-31. [Crossref] [PubMed]
- Perez J, Chavarriaga J, Ortiz A, et al. Oncological and Functional Outcomes After Organ-Sparing Plastic Reconstructive Surgery for Penile Cancer. Urology 2020;142:161-5.e1. [Crossref] [PubMed]


