
Activation of the iNOS/NO/cGMP pathway by Revactin® in human corporal smooth muscle cells
Introduction
Erectile dysfunction (ED), defined as the inability to attain or sustain an erection during sexual intercourse, is estimated to affect approximately 52% of men aged 40–70 years (1). The predominant cause of ED, regardless of age, appears to be cavernosal veno- occlusive dysfunction (CVOD) or venous leakage (2), which is primarily due to the progressive loss of the CSMC, leading to a relative increase in cavernosal fibrosis (3,4). It is estimated that CVOD becomes clinically apparent when there is a loss of approximately 10–20% of the corporal smooth muscle cells (HCSMC) (5,6).
The current treatments available for men with ED, while they may allow men to get an on-demand erection, are simply palliative and do not target the cause of the dysfunction.
The phosphodiesterase (PDE) type 5 inhibitors such as sildenafil, tadalafil, etc., are usually the first-line treatment for men with ED. However, they are only effective in producing a satisfactory erection in about 60–75% of men who initially try it (7-9); however, over time, the majority of these men who initially respond to these drugs will ultimately fail this treatment (10). This failure is definitely not due to tachyphylaxis of the drug (11,12) but is presumed to be due to the progressive ongoing aging related apoptosis of the HCSMC (13). It has been suggested that when such aging-related apoptosis occurs, it appears to be associated with an increase in intracellular oxidative stress within the corporal tissue (14). In an attempt to counteract the oxidative stress causing this apoptotic process, the CSMC themselves begin to produce nitric oxide (NO) via the normally inactive inducible nitric oxide synthase (iNOS) enzyme (15-17).
The NO emanating from this endogenous process within the CSMC has been shown to neutralize oxidative stress and reduce the ongoing apoptotic process (15).
It was recently reported in the rat that the oral administration of a nutraceutical composition (COMP-4) consisted of muira puama, ginger rhizome, and Paullinia cupana together with the amino acid, L-citrulline, reversed the corporal apoptosis and fibrosis as well as corrected the CVOD observed in the aged rat (18). The proposed mechanism of action of COMP-4 was activation of the relatively dormant iNOS-NO-cGMP pathway within the CSMC (19). This increase in NO and cGMP via iNOS was postulated to promote the reduction in apoptosis and fibrosis as well as the improvement in the erectile function observed in the aging rat penis (18).
Commercially, COMP-4 is marketed as Revactin®, and while slightly different in its composition from COMP-4, it was employed in a 3-month pilot clinical trial that showed a significant early improvement in erectile function (20,21). It was theorized at that time that the improvement in erectile function in these men could possibly be due to the putative elevation in levels of cGMP produced via the activation of the iNOS-cGMP pathway.
In order to answer the question of whether the improvement in erectile function observed in men taking Revactin® could be ascribed to the activation of the endogenous iNOS-cGMP pathway in the HCSMC, a study using a human primary CSM in vitro model was performed to evaluate the effect of Revactin® on cGMP and nitrite formation as well as its effect on the nitric oxide synthases (nNOS, eNOS, and iNOS).
We present the following article in accordance with the MDAR checklist (available at https://dx.doi.org/10.21037/tau-21-11).
Methods
Cell cultures
Primary HCSMC were initiated from snippets of corporal tissue removed from male patients (n=4) undergoing an invasive penile surgical procedure on the corpora cavernosa. The study was conducted in agreement with the Declaration of Helsinki (as revised in 2013), and it was approved as exempt by the UCLA IRB #19-001074, and by the CDU IBC #18-07-0034-01-03. The individual’s consent for this study was waived.
The corpora cavernosa explants were attached to a T75-cm2 flask incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal calf serum and 1X antibiotic-antimycotic solution (Corning Technology, Corning NY Cat# 30-004-CI) (19,22). The tissue explants were cultured at 37 °C in a 5% CO2 incubator for a week, avoiding the tissue’s dislodgment. The tissue was removed when the cells started migrating from the tissue. After trypsinization, cells were then transferred to a new T75-cm2 flask at a ratio of 0.5×106–1.0×106 cells per flask and grown in human smooth muscle growth medium (Cell Applications Inc; Cat# 311-500, San Diego CA) until reaching confluency at 8.0×106 cells per flask. For the experiments, cells between the second and fifth passages were seeded in 6-wells plates at 0.3×106 cells until they reached confluency at 1.0×106 to start the treatments.
Preparation of Revactin®
Revactin® was a gift of MD Concepts (New York, NY). The normal daily dose of Revactin® in human is 500 mg each of muira puama, ginger rhizome, and Paullinia cupana, the latter containing 12.0% caffeine, as well as 1,600 mg of L-citrulline. In order to determine which concentration of Revactin® would be safe for cell viability, and to correlate these experiments with the dosage of Revactin® used in the aforementioned clinical studies (20,21), three different concentrations of Revactin®, were prepared at 50% (0.345 mg/mL), 100% (0.69 mg/mL), and 200% (1.38 mg/mL). The concentrations used in the cell culture experiments correlate with the above-mentioned human dose of Revactin® (20,21). The contents within the Revactin® capsule were dissolved in 0.07% ethanol.
The phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX; cat# 13347), the phosphodiesterase V inhibitor, Sildenafil (SIL; Cat# 139755-83-2), and the iNOS specific inhibitor, N6-(1-iminoethyl)-L-lysine, dihydrochloride (L-NIL; cat#: 80310) were obtained from Cayman Chemical Company (Ann Arbor, MI) and used at a concentration of 1 mM for IBMX, 0.4 mM for SIL, and 4 μM for L-NIL, respectively.
Immunocytochemistry of alpha smooth muscle actin and desmin
HCSMC were seeded at 40,000 cells per well in four-well Nunc® Lab-Tek® chamber slides (Thermo Fisher, Waltham MA) until they reach confluency. Cells were fixed with 10% formalin for 20 minutes. After quenching for endogenous peroxidase activity with 3% H2O2, cells were permeabilized with 0.3% Triton in PBS and then blocked with 10% normal horse serum or normal goat serum in 0.3% Triton-PBS. Cells were then incubated with: (I) a monoclonal antibody against alpha-smooth muscle actin at a dilution of 1:400 (Sigma Aldrich Cat#: A5228, RRID: AB_262054, St Louis, MO, USA), and (II) a polyclonal antibody made in rabbit against desmin at 1:400 dilution (AbCAM Cat#: ab32362 RRID:AB_731901, Cambridge, UK). After several washes with PBS, an incubation with 1:200 dilution of either horse anti-mouse (Cat# BA-2000 RRID:AB_2313581) or goat anti-rabbit (Cat# BA-1000, RRID:AB_2313606) biotinylated secondary antibodies from Vector Laboratories (Burlingham, CA, USA) was performed, followed by the ABC complex in 1:100 dilution (Vectastain Elite ABC System Cat#: PK 6100, Vector Laboratories). As chromogen, 3-amino-9-ethylcarbazole in buffer acetate and 0.06% of H2O2 (Sigma Aldrich; Cat#: AEC 101) was used. Hematoxylin was used as a counterstaining. Slides were detached from the chambers, and a coverslip was mounted with aqua-mount mounting media (Vector Laboratories; Cat#: H5501). The first antibody was omitted as negative control (23,24).
Determination of cGMP
The HCSMC were seeded at 0.3×106 cells per well in a 6-well plate, and after reaching confluency, cells were incubated with vehicle or with different concentrations of Revactin® for 24 hours. A time course at 0, 3, 6, 12, and 24 hours incubation with 100% of Revactin® and with 0.4 mM sildenafil as a positive control was also performed.
The incubations with the treatments and inhibitors were stopped by removing the media and the addition of HCL 0.1 M for 20 minutes. Cells were then homogenized and centrifuged at 1,000 g for 10 minutes. The supernatants were used for the determination of cGMP concentration by a colorimetric ELISA (Cayman Chemical Company, Cat#: 581021, Ann Arbor, MI), following the manufacturer’s instructions without acetylation. The results were expressed as pmol/mg protein (22).
Determination of nitrite formation
After 24 hours of incubation with the vehicle and the treatments, the cell culture media was collected and frozen at −20 °C until the measurement of the total nitrite concentration by the Griess Reaction (Cayman Chemical Company; Cat#: 780001). Briefly, nitrite blanks, standards, and the sample media were incubated with Griess reagent 1, followed by Griess reagent 2. After incubating for 10 minutes at RT, the absorbance was measured at 540 nm. The nitrite concentration in the media samples was expressed in micromolar (µM) (22).
Western blotting and densitometry analysis
After the incubation with the vehicle and the treatments, cell lysates containing 50 ug of protein were separated using 4–15% Tris-HCl PAGE precast gels (Bio-Rad, Hercules, CA) in Tris/glycine/SDS running buffer. The separated proteins were electrophoretic transferred onto polyvinylidene fluoride (PVDF) membranes in Tris/glycine/methanol transfer buffer using transblot semi-dry apparatus (Bio-Rad, Hercules, CA, USA). Membranes were blocked in 5% non-fat milk with 0.1% Tween20 in PBS for one hour at RT, as it was previously described (19,22). After several washes with the PBS Tween 0.1% washing buffer, membranes were incubated overnight at 4 °C with the primary antibodies for (I) iNOS at 1:250 dilution (Abcam Cat# ab15323, RRID:AB_301857 Cambridge, UK); (II) eNOS at 1:500 dilution (BD Biosciences Cat# 610299, RRID:AB_397693 San Jose, CA, USA); (III) nNOS at 1:500 dilution (Abcam Cat# ab76067, RRID:AB_2152469); (IV) the amino acid transporter SLC38A1/NAT2 at 1:800 dilution (Abcam Cat# ab134268, RRID:AB_945505). GAPDH at 1:5000 dilution was used as a loading control (Millipore Cat# MAB374, RRID:AB_2107445, Billerica, MA, USA). After several washes with buffer, the membranes were incubated for 2 hrs at RT with 1:2,000 dilution of anti-mouse or anti-rabbit secondary antibody linked with HRP (Cell Signaling Technology, Cat# 7076, RRID:AB_330924 and Cat# 7074, RRID:AB_2099233, Danvers, MA, USA). The immunoreactive bands were visualized using a chemiluminescent detection system, WesternSure PREMIUM (Li-COR Biotechnology Cat# 926-95000, Lincoln, NE, USA). The bands were scanned with C-DiGit Blot Scanner (Li-COR Biotechnology) and analyzed with the Image Studio Software, version 5.2 (Li-COR Biotechnology).
Statistical analysis
All data are presented as mean ± S.E.M. Differences between groups were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test using GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, CA). All comparisons were two-tailed, and P<0.05 were considered statistically significant. Cells were seeded in 6-well plates at 0.3 106 cells per well or four-well chambers at 40,000 cells per well using simple randomization. All in vitro experiments were repeated at least thrice from four different patients (n=4), and the data from representative experiments are shown.
Results
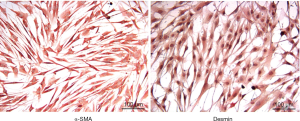
In order to determine the purity of the HCSMC primary cell culture, immunohistochemistry was performed with two smooth muscle cell markers, alpha smooth muscle actin and desmin. Figure 1 shows a strong positive expression of both markers in which 100% of the cells in each well expressed both alpha smooth muscle actin and desmin indicating that the primary cell culture only contained smooth muscle cells (25). This corroboration of the positive staining for α-smooth muscle actin and desmin was done in all the patients in order to maintain the integrity of the study.
To determine whether Revactin® was capable of stimulating NO production in the HCSMC, nitrite formation was measured in the medium after the cells were incubated with different concentrations of Revactin®. Figure 2A shows that the 50% Revactin® dose increased nitrite production by 30.5% (P=0.0247); the 100% dose of Revactin® increased it by 74% (P<0.0001); and the 200% dose of Revactin® increased it by 61% (P=0.0003), when compared with the control. As expected, the PDE inhibitor IBMX did not stimulate nitrite formation.
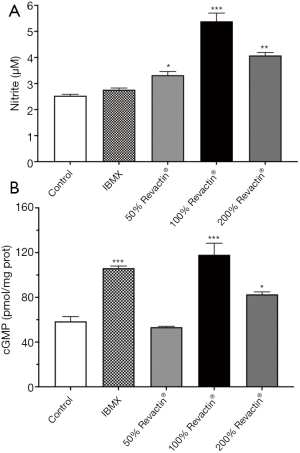
To ascertain whether the increase in nitrite formation, a surrogate for NO production, was translated into the activation of the endogenous NO-cGMP pathway in the HCSMC, cGMP production was determined similarly with the 50%, 100%, and 200% concentrations of Revactin®. As seen in Figure 2B, after 24 hours of incubation and when compared to the control, there was no significant difference in cGMP (P=0.8873) production with the 50% concentration of Revactin®. However, with the 100% Revactin® concentration, there was a significant 2.0-fold increase in the production of cGMP (P<0.0001), while with the 200% Revactin® dose, there was only a 41% increase in cGMP production (P=0.0266). When the CSMC were incubated with IBMX which was used as our positive control, there was a 1.8-fold increase in cGMP production (P=0.0003).
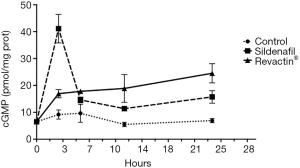
To corroborate that the time of the incubation with 100% Revactin® for 24 hours was the one that provided the highest cGMP production, a time course was performed in the HCSMC. Sildenafil was used as a positive control of cGMP expression. Figure 3 shows that while the cGMP expression was the highest for sildenafil at 3 hours of incubation, Revactin® has a steady increase throughout the time course, reaching the maximum concentration of cGMP at 24 hours.
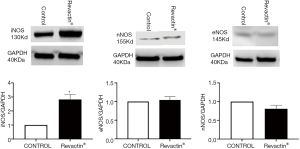
To determine whether the increase in NO production observed with Revactin® could be due to changes in the expression of any of the three endogenous nitric oxide synthases within the HCSMC, western blots were performed on these HCSMC treated with the 100% dose of Revactin®. Figure 4 shows that this dose of Revactin® significantly increased iNOS expression by 2.9-fold (P=0.0102) with respect to the control. The expression of the other two NOS synthases, eNOS and nNOS, were not upregulated by Revactin®.
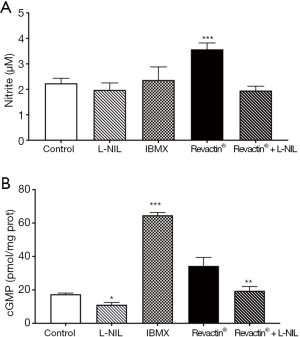
To demonstrate unequivocally that the effect of Revactin® is via its activation of iNOS, we co-incubated the 100% dose of Revactin® with L-NIL, a specific inhibitor of iNOS activity. Figure 5A shows that L-NIL decreased nitrite formation by 45% (P=0.018) and Figure 5B shows that it decreased cGMP production by 43%, when compared to its control.
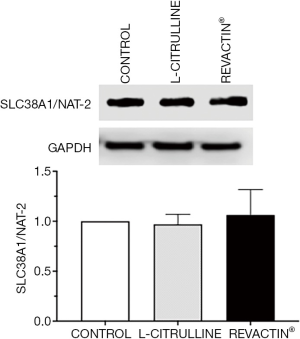
To demonstrate that the amino acid L-CIT, one of the components of Revactin, is capable of entering the HCSMC, the expression of the neutral amino acid transporter responsible for this action was evaluated by western blot. Figure 6 shows that amino acid transporter SLC38A1/NAT2 is expressed in our HCSM cell culture, and the addition of L-CIT or Revactin® did not modify its expression.
Discussion
This study demonstrates that Revactin® is capable of activating the normally dormant iNOS-NO-cGMP pathway within the HCSMC. In this in vitro study, exposure of the HCSMC to Revactin® for 24 hours resulted in an increase in the expression of iNOS and NO as well as an increase in the intracellular formation of cGMP. The NO-cGMP response to Revactin® is presumed to be solely iNOS dependent since incubation with the product increased the content of iNOS but not eNOS nor nNOS. In addition, L-NIL, a specific inhibitor of iNOS, was capable of completely blocking the formation of cGMP by Revactin®. The increase in iNOS expression observed with Revactin® is probably due to either a modulation of the mRNA levels of iNOS, similar to what we have observed previously in the rat CSMC (19), or to post-trancriptional modifcations that would lead to an increase in the protein expression of iNOS.
The cGMP response to Revactin® appears to be dose dependent in that the maximum formation of cGMP in vitro occurred when the HCSMC were exposed to the corresponding recommended daily human dose which comprises 500 mg each of ginger rhizome, muira puama and Paullinia cupana combined with approximately 1,600 mgof L-citrulline (20,21). Even though 50%, 100% and 200% Revactin® doses are all capable of inducing NO production by the HCSMC as is evidenced in our assay by the formation of nitrite (Figure 2A), a known footprint for NO, it was only the 100% and 200% doses that resulted in significant cGMP formation. Such an observation would indicate that a certain level of NO may be required to activate the soluble guanylyl cyclase (sGC) enzyme for the production of cGMP and several studies have shown that a high level of NO (250–1,600 nM) is actually required to produce half-maximal activity of this sGC enzyme (26). This could explain why the 100% and 200% but not the 50% Revactin® doses were capable of promoting cGMP production. This dose dependency of the HCSMC to Revactin® parallels what was previously observed in a similar study in the rat where COMP-4, the four main constituents of Revactin®, was used to treat the cells singly or in combination (19).
It has been theorized that when the pre-determined aging related changes that impact corporal smooth muscle relaxation begin to occur in the CSMC most likely due to the onset of oxidative stress, the CSMC themselves begin to counteract this stress by initiating the production of NO intracellularly via this normally dormant iNOS enzyme (17). The NO being produced by iNOS in such a scenario has a dual purpose: (I) to combat this oxidative stress by directly neutralizing within the mitochondria the newly formed reactive oxidation species (ROS) and (II) to form cGMP which begins a series of processes to repair the cellular changes that have occurred as a result of the damage done to the cellular architecture by the oxidative stress. In the aged rat, it was reported that such long-term daily treatment with the combination of these four constituents of Revactin® not only resulted in a marked improvement in the histology of the corpora (18) but it was determined that the response of the erectile tissue of these aged rats to pharmacological stimulation reverted to what is normally seen in much younger animals (18). In addition to the improvement in these histological and functional metrics of these treated aged animals, it was determined from measuring the GSH/GSSG ratio in the blood that the systemic oxidative stress of these treated aged animals was also markedly reduced (18) as evidenced by (I) the reduction in apoptotic activity and (II) no changes in the nitrotyrosine expression not only within the CSMC but also within the smooth muscle cells of the arterial media (27). This suggests that the combination of the products found in Revactin® when ingested long-term may play a salutary role in combating systemic oxidative stress as well as stimulating those aged cells to begin repairing itself. This is somewhat similar to what has been shown to occur in a bone fracture model where the production of NO, presumed to emanate from iNOS, accelerated fracture healing (28,29) while the deletion of iNOS in this experimental model resulted in an impairment in the healing of these fractures (30). Besides its role in the bone and corporal tissue, a beneficial effect of iNOS has also been implicated in the process of repairing the intestinal mucosa after chronic injury (31,32). In addition, it has been shown that delivery of iNOS to the skin using adenoviral vectors improved healing of this tissue in iNOS−/− mice (33), suggesting that iNOS may be therapeutically helpful if given in appropriately regulated amounts at specific sites (32).
Since our results show that Revactin® does not appear to modulate the expression of nNOS and eNOS within the HCSMC, this would suggest that the presumptive anti-oxidative, anti-fibrotic and anti-apoptotic effects of Revactin® is primarily the result of NO expression from iNOS (15). Furthermore, it also suggests that the improvement in erectile function previously reported in those men who took Revactin (20,21) could most likely have been due to the production of NO via the upregulation of the endogenous iNOS-NO-cGMP pathway within the corporal smooth muscle itself and not due to any effect from eNOS nor nNOS.
Revactin® consists of four constituents, each demonstrating some individual involvement in either the NOS-NO-cGMP pathway or corporal smooth muscle relaxation or both (19). For example, specific forms of ginger at certain doses have been shown to stimulate iNOS and NO in RAW 264.7 cells (34), muira puama has been shown to stimulate the sGC enzyme, the enzyme that converts GTP to cGMP (35) while Paullinia cupana is known to increase cAMP rather than cGMP levels (35), as well as inhibit the synthesis of the PDE enzyme (19).
One of the limitations of this study is that although we have assumed that the production of NO from iNOS by Revactin® could reduce oxidative stress within these HCSMC, such changes were not measured directly within the cell itself following incubation with the Revactin®. In addition, it was not determined whether Revactin® had any effect on the expression of the PDE5 enzyme in these HCSMC although, Paullinia cupana, one of the constituents of the product has been shown in the rat CSMC to inhibit the expression of the PDE5 enzyme (19).
In summary, this in vitro study suggests that stimulation of the iNOS-NO-cGMP pathway within the HCSMC by Revactin® may play a role in combating the aging related deterioration of these cells resulting from the oxidative stress that is part and parcel of the normal aging process. It is obvious that appropriately designed human clinical trials will be necessary to determine this.
Acknowledgments
Funding: This study was funded in part by a grant from the Peter A Morton Foundation (JR). Certain aspects of the study were also supported in part by the NIH-National Institute on Minority Health and Health Disparities (NIH/NIMHD) 5U54MD007598-06 (MGF).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at https://dx.doi.org/10.21037/tau-21-11
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tau-21-11
Peer Review File: Available at https://dx.doi.org/10.21037/tau-21-11
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-11). Dr. MGF reports a grant from NIH-National Institute on Minority Health and Health Disparities, during the conduct of the study. Dr. JR reports a grant from the Peter Morton Foundation and a stockholder in KLRM, LLC. KLRM, LLC is the assignee for the patent of COMB-4/COMP-4. MD Concepts pays KLRM, LLC a royalty on sales of Revactin®. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved as exempt by the UCLA IRB #19-001074 and approved by the CDU IBC #18-07-0034-01-03, and individual consent for this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feldman HA, Goldstein I, Hatzichristou DG. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol 1988;140:69-71. [Crossref] [PubMed]
- Jevtich MJ, Khawand NY, Vidic B. Clinical significance of ultrastructural findings in the corpora cavernosa of normal and impotent men. J Urol 1990;143:289-93. [Crossref] [PubMed]
- Sattar AA, Merckx LA, Wespes E. Penile electromyography and its smooth muscle content: interpretation of 25 impotent patients. J Urol 1996;155:909-12. [Crossref] [PubMed]
- Nehra A, Goldstein I, Pabby A, et al. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol 1996;156:1320-9. [Crossref] [PubMed]
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers 2016;2:16003. [Crossref] [PubMed]
- Padma-Nathan H, Steers WD, Wicker PA. Efficacy and safety of oral sildenafil in the treatment of erectile dysfunction: a double-blind, placebo-controlled study of 329 patients. Sildenafil Study Group. Int J Clin Pract 1998;52:375-9. [PubMed]
- Porst H, Rosen R, Padma-Nathan H, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res 2001;13:192-9. [Crossref] [PubMed]
- Eardley I. Vardenafil: a new oral treatment for erectile dysfunction. Int J Clin Pract 2004;58:801-6. [Crossref] [PubMed]
- El-Galley R, Rutland H, Talic R, et al. Long-term efficacy of sildenafil and tachyphylaxis effect. J Urol 2001;166:927-31. [Crossref] [PubMed]
- Musicki B, Champion HC, Becker RE, et al. In vivo analysis of chronic phosphodiesterase-5 inhibition with sildenafil in penile erectile tissues: no tachyphylaxis effect. J.Urol 2005;174:1493-6. [Crossref] [PubMed]
- Vernet D, Magee T, Qian A, et al. Phosphodiesterase type 5 is not upregulated by tadalafil in cultures of human penile cells. J Sex Med 2006;3:84-94. [Crossref] [PubMed]
- Ferrini M, Magee TR, Vernet D, et al. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod 2001;64:974-82. [Crossref] [PubMed]
- Pollack M, Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci 2001;56:B475-82. [Crossref] [PubMed]
- Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide 2002;6:283-94. [Crossref] [PubMed]
- Ferrini MG, Rivera S, Moon J, et al. The genetic inactivation of inducible nitric oxide synthase (iNOS) intensifies fibrosis and oxidative stress in the penile corpora cavernosa in type 1 diabetes. J Sex Med 2010;7:3033-44. [Crossref] [PubMed]
- Ferrini MG, Gonzalez-Cadavid NF, Rajfer J. Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl Androl Urol 2017;6:20-7. [Crossref] [PubMed]
- Ferrini MG, Hlaing SM, Chan A, et al. Treatment with a combination of ginger, L-citrulline, muira puama and Paullinia cupana can reverse the progression of corporal smooth muscle loss, fibrosis and veno-occlusive dysfunction in the aging rat. Andrology (Los Angel) 2015;4:e132 [Crossref] [PubMed]
- Ferrini MG, Garcia E, Abraham A, et al. Effect of ginger, Paullinia cupana, muira puama and l- citrulline, singly or in combination, on modulation of the inducible nitric oxide- NO-cGMP pathway in rat penile smooth muscle cells. Nitric Oxide 2018;76:81-6. [Crossref] [PubMed]
- Nguyen S, Rajfer J, Shaheen M. Safety and efficacy of daily Revactin® in men with erectile dysfunction: a 3-month pilot study. Transl Androl Urol 2018;7:266-73. [Crossref] [PubMed]
- Nguyen S, Shaheen M, Pak Y, Rajfer J. Early improvement in SHIM scores with Revactin®. Int J Impot Res 2020;32:255-6. [Crossref] [PubMed]
- Ferrini MG, Abraham A, Nguyen S, et al. Exogenous L-Arginine does not stimulate production of NO or cGMP within the rat corporal smooth muscle cells in culture. Nitric Oxide 2019;89:64-70. [Crossref] [PubMed]
- Elliott KJ, Eguchi S. In Vitro Assays to Determine Smooth Muscle Cell Hypertrophy, Protein Content, and Fibrosis. Methods Mol Biol 2017;1614:147-53. [Crossref] [PubMed]
- Braga M, Simmons Z, Norris KC, et al. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr Connect 2017;6:139-50. [Crossref] [PubMed]
- Rangdaeng S, Truong LD. Comparative Immunohistochemical Staining for Desmin and Muscle-Specific Actin: A Study of 576 Cases. Am J Clin Pathol 1991;96:32-45. [Crossref] [PubMed]
- Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 2008;10:1185-98. [Crossref] [PubMed]
- Nguyen S, Castellanos KA, Abraham A, et al. Reduction of oxidative stress markers in the corpora cavernosa and media of penile dorsal artery in middle-aged rats treated with COMP-4. Int J Impot Res 2021;33:67-74. [Crossref] [PubMed]
- Rajfer RA, Kilic A, Neviaser AS, et al. Enhancement of fracture healing in the rat, modulated by compounds that stimulate inducible nitric oxide synthase: Acceleration of fracture healing via inducible nitric oxide synthase. Bone Joint Res 2017;6:90-7. [Crossref] [PubMed]
- Diwan AD, Wang MX, Jang D, et al. Nitric oxide modulates fracture healing. J Bone Miner Res 2000;15:342-51. [Crossref] [PubMed]
- Baldik Y, Diwan AD, Appleyard RC, et al. Deletion of iNOS gene impairs mouse fracture healing. Bone 2005;37:32-6. [Crossref] [PubMed]
- McCafferty DM, Mudgett JS, Swain MG, et al. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology 1997;112:1022-7. [Crossref] [PubMed]
- Kubes P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut 2000;47:6-9. [Crossref] [PubMed]
- Yamasaki K, Edington HD, McClosky C, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest 1998;101:967-71. [Crossref] [PubMed]
- Imanishi N, Mantani N, Sakai S, et al. Inducible activity of ginger rhizome (Zingiber officinale Rosc.) on the mRNA expression of macrophage-inducible nitric oxide (NO) synthase and NO production in a macrophage cell line, RAW264.7 cells. Am J Chin Med 2004;32:727-35. [Crossref] [PubMed]
- Antunes E, Gordo WM, de Oliveira JF, et al. The relaxation of isolated rabbit corpus cavernosum by the herbal medicine Catuama and its constituents. Phytother Res 2001;15:416-21. [Crossref] [PubMed]


