
Expression and clinical significance of NUF2 in kidney renal clear cell carcinoma
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3% of all systemic malignancies and 85–90% of primary malignancies in the adult kidney. It is the second-most common urological malignancy, with kidney renal clear cell carcinoma (KIRC) accounting for 85% of the cases (1). The cytokinesis-related gene, NUF2 (Ndc80 kinetochore complex component) is involved in mitotic granule stabilization and proper chromosome segregation during cell mitosis (2), and has been reported to be highly expressed in a variety of malignancies and playing a key role in tumor formation and progression (3). However, to date, NUF2 has not been reported in KIRC, and its mechanisms of action remain unclear. Therefore, in the current study, The Cancer Genome Atlas (TCGA) database and pathological specimens from the First People's Hospital of Taicang were used to investigate the expression and clinical significance of this gene in KIRC, as well as its possible regulatory pathways. We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/tau-21-620).
Methods
Collection of data
The mRNA data and corresponding clinical data of 537 patients with KIRC and 72 normal control patients were obtained from the TCGA database. Cases were divided into a high expression group and a low expression group based on whether the expression was greater than or less than the mean. The expression of NUF2mRNA in KIRC and its relationship with overall survival (OS), progression-free survival (PFS), and clinical pathological characteristics were analyzed.
Case selection
Eighty-three KIRC patients who underwent urology surgery at Taicang Hospital of Soochow University between January 2014 and December 2020 were selected, which included 52 males and 31 females with a mean age of 61 years. Pathological diagnosis revealed that 71 patients were in stage I, five patients were in stage II, seven were in stage III, 21 were in grade G1, 57 were in grade G2, and five patients were in grade G3. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First People’s Hospital of Taicang (NO. 2021-KY-161, the registration number of ethics board) and informed consent was taken from all the patients.
Immunohistochemical analysis
Immunohistochemical staining was performed using a Roche BenchMarkGX semi-automatic immunohistochemistry machine to detect cancer and paired para-cancerous tissues. All paraffin specimens were subjected to 4-µm sectioning, and two consecutive slices were cut and baked in an oven at 65 °C for 30. This thin section was then subjected to HE staining to obtain a detailed view of the tissues.
Evaluation and analysis of the results
Positive staining was characterized as yellowish-brown staining of the cytoplasm. Five fields were randomly selected at high magnification, and the intensity of positive staining was scored as follows: cells-stained light yellow received 1 point; cells stained yellow received 2 points, and cells-stained brown received 3 points. The percent of positive-stained cells was evaluated as follows: 0 points equals <10%; 1 point equaled 11–25%; 2 points equaled 26–50%; 3 points equaled 51–75%; and 4 points equaled >75%. The immunohistochemistry score was determined by multiplying the two scores. Correlation between the immunohistochemistry scores and the clinicopathological characteristics of patients was then investigated.
GSEA analysis
To analyze the possible regulatory pathways of the NUF2 gene in the development of KIRC, GSEA analysis (V4.0.3) was employed.
Statistical processing
R (V3.62) software was used for data processing, and the Beeswarm plugin was used to analyze variations in NUF2 mRNA expression. The Kaplan-Meier method was used to perform survival analysis, and the relationship between NUF2 expression levels and clinicopathological characteristics was analyzed by Kruskal Walls test and logistic regression. Correlation analysis was carried out using Pearson rank correlation, and the relationship between NUF2 mRNA levels, clinicopathological characteristics, and OS was analyzed by univariate and multifactorial Cox regression. P<0.05 was considered statistically significant.
Results
KIRC patient characteristics
Detailed clinicopathological characteristics data of KIRC patients from the TCGA database are shown in Table 1.
Table 1
| Clinical characteristic | N |
|---|---|
| Age | |
| >60 years | 271 |
| ≤60 years | 266 |
| Gender | |
| Male | 346 |
| Female | 191 |
| Stage | |
| I | 269 |
| II | 57 |
| III | 125 |
| IV | 83 |
| Tumor | |
| T1 | 275 |
| T2 | 69 |
| T3 | 182 |
| T4 | 17 |
| Lymph nodes metastasis | |
| N0 | 240 |
| N1 | 11 |
| Grade | |
| G1 | 14 |
| G2 | 230 |
| G3 | 207 |
| G4 | 78 |
| Distant metastasis | |
| M0 | 426 |
| M1 | 79 |
NUF2 mRNA expression in KIRC patients and relationship with OS
NUF2 mRNA levels were significantly increased in KIRC patients as compared to control patients (Figure 1). Levels in KIRC patients were significantly associated with survival, and the prognosis of patients with high NUF2 mRNA expression was significantly worse than that of patients with low expression (Figure 2).
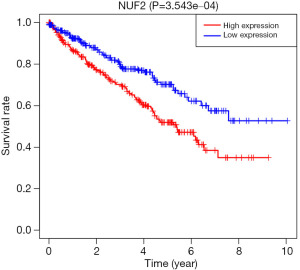
Relationship between NUF2 mRNA expression and clinicopathological characteristics
Statistically significant differences in NUF2 mRNA expression were found between patients with different AJCC stage, different T stage, presence or absence of lymph node metastases, presence or absence of distant metastases, and different gender (Figure 3). Logistic regression analysis also suggested that NUF2 mRNA expression was associated with patient stage, T stage, lymph node metastases, and distant metastases and gender (Table 2).
Table 2
| Clinical characteristic | N | Odds ratio | P |
|---|---|---|---|
| Stage (IV |
352 | 3.314 (1.970–5.709) | 9.430E-6 |
| Grade (4 |
92 | 6.519 (1.849–30.704) | 0.007 |
| Tumor (4 |
286 | 14.862 (2.788–274.722) | 0.011 |
| Lymph nodes metastasis | 257 | 4.751 (1.486–21.109) | 0.007 |
| Distant metastasis | 505 | 2.786 (1.671–4.778) | 1.338E-4 |
| Age | 537 | 0.988 (0.974–1.002) | 0.087 |
| Gender | 537 | 1.541 (1.077–2.212) | 0.018 |
Results of COX regression analysis
Univariate COX analysis demonstrated that OS was associated with age, stage, T-stage, lymph node metastases, distant metastases, and NUF2 mRNA expression. Multifactorial Cox analysis showed that NUF2 mRNA level, age, AJCC stage, and pathological grade were independent prognostic factors for OS, (Table 3 and Figure 4). Only 489 patients had complete clinicopathological data for analysis. The effect of lymph node metastasis status on survival was excluded from the COX regression analysis as many patients had an unknown lymph node metastasis status.
Table 3
| Parameter | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 1.762 | 1.019–2.420 | 4.81E-04 | 1.667 | 1.208–2.230 | 0.002 | |
| Gender | 0.874 | 0.629–1.214 | 0.421 | 1.048 | 0.749–1.466 | 0.786 | |
| Grade | 2.293 | 1.854–2.836 | 1.94E-14 | 1.408 | 1.108–1.791 | 0.005 | |
| Stage | 1.889 | 1.649–2.164 | 4.67E-20 | 1.567 | 1.002–2.452 | 0.049 | |
| Tumor | 1.941 | 1.639–2.299 | 1.50E-14 | 0.896 | 0.594–1.351 | 0.600 | |
| Distant metastasis | 4.284 | 3.10–5.908 | 7.45E-19 | 1.411 | 0.719–2.70 | 0.317 | |
| NUF2 | 1.353 | 1.258–1.454 | 3.40E-16 | 1.257 | 1.57–1.367 | 7.90E-8 | |
Expression of NUF2 protein in KIRC patients
Paired analysis of 83 KIRC patients showed that the mean levels of NUF2 protein expression in cancer and para-cancerous tissues were 6.06±3.16 and 2.55±1.04 points, respectively, and the difference between the two was statistically significant (Figures 5,6).
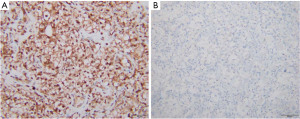
Relationship between NUF2 protein expression and clinicopathological characteristics in KIRC patients
The mean expression of NUF2 protein in KIRC patients was 4.67±2.71, 6.47±3.21, and 7.20±3.03 points in patients with G1, G2, and G3 levels, respectively, with statistically significant differences (Figure 6). The mean expression of NUF2 protein in patients with KIRC at stages I, II, and III was 6.04±3.20, 8.20±2.49, and 4.71±2.50 points, respectively, with no statistically significant difference (Figure 7). NUF2 protein expression was significantly correlated with pathological grading (r=0.226, P=0.017), but not with stage (r=0.009, P>0.05).
Regulatory pathways associated with NUF2 mRNA
GSEA analysis indicated that high expression of NUF2 was associated with the upregulation of pathways such as homologous recombination, DNA replication, cell cycle regulation, mismatch repair, and the P53 signaling pathway (Figure 8). High expression of NUF2 mRNA was associated with the downregulation of pathways such as propanoate metabolism, pyruvate metabolism, the citrate cycle TCA cycle, butanoate metabolism, and the degradation of valine, leucine, and isoleucine (Figure 9).
Discussion
RCC is a common urological malignancy. In the year 2015, the number of new cases of RCC in China was 66,800 (43,200 for men and 23,600 for women), accounting for 1.56% of all new malignant tumor cases and ranking 14th among all malignant tumor cases. The number of deaths from the disease was 23,400 (15,200 for men and 8,200 for women), accounting for 0.83% of all malignant tumor deaths and ranking 17th among all malignant tumor deaths (4). Although surgical resection is the first line of therapy for RCC, up to 40% of patients have a local recurrence and distant metastases (5). To increase patient survival rates, biological markers that allow for early diagnosis and prognosis are critical, and in this research KIRC was explored as it is the most common histological type of RCC.
A major characteristic of all tumor cells is genomic instability (6). The abnormal expression of NUF2 can lead to mitotic dysregulation, which can contribute to the development of tumors (7). Sun et al. found that NUF2 was significantly expressed in breast cancer in comparison to normal breast tissue, and that a high expression group had lower OS and recurrence-free survival than a low expression group (8). They concluded that NUF2 could be utilized as a biomarker to identify patients with a poor prognosis. Other studies have found that the growth of pancreatic, glioma, and hepatocellular carcinoma tumor cells could be inhibited by knocking down the expression of NUF2 (9-11). From this current study of TCGA data, it was found that NUF2 mRNA levels were significantly higher in KIRC patients than in controls, and the prognosis of patients with high NUF2 mRNA expression was significantly worse than that of patients with low expression. Statistically significant differences in NUF2 mRNA expression were found between patients with different AJCC stages, different T stages, in the presence or absence of lymph node metastases, and presence or absence of distant metastases. Logistic regression analysis also suggested that NUF2 mRNA expression was associated with patient stages, T stage, lymph node metastases, and distant metastases. Collectively, these results suggest that NUF2 might play an important role in the occurrence and development of KIRC. Multifactorial COX regression analysis showed that NUF2 mRNA was an independent prognostic risk factor, suggesting NUF2 may serve as an indicator for the prognosis and diagnosis of KIRC. At present, there is no widely accepted prognosis biomarker for KIRC, so NUF2 maybe one of the potential effective prognostic marker for KIRC. Recent studies have also found some KIRC prognostic factors such as: androgen receptors (AR), miR-106b-5p, etc. (12). Combining NUF2 with these factors will likely provide more accurate prognostic predictions for KIRC.
To validate the expression of NUF2 mRNA in Chinese KIRC patients, an immunohistochemical study of KIRC specimens from 83 patients was performed and revealed that NUF2 protein expression was significantly higher in KIRC cancer tissues than in paired para-cancerous tissues. In addition, NUF2 protein expression was significantly correlated with the pathological grades of patients. These protein level findings suggest that the NUF2 gene may contribute to the occurrence and development of KIRC and can be regarded as an indicator of patient prognosis. However, unlike prior research on NUF2 mRNA, our findings did not show that the AJCC stage was linked to NUF2 protein expression. This may be due to: (I) the small sample size, and that most patients were stage I; (II) although NUF2 mRNA expression level was related to AJCC stages, after translation, post-translational processing, etc., the protein expression level of NUF2 was indeed not related to stages. In turn, because of these factors, most patients had not yet relapsed and died, so the relationship between NUF2 protein and patient survival could not be examined. Research involving a larger sample size and longer follow-up is essential to verify the results.
GSEA analysis was performed to further explore the possible regulatory pathways of NUF2 in the development of KIRC and revealed that high expression of NUF2 was related to the upregulation of pathways such as homologous recombination, DNA replication, cell cycle regulation, mismatch repair, and the P53 signaling pathway. Tumor cells are known to exhibit more chromosomal alterations compared to normal cells, and upregulation of homologous recombination can lead to genetic instability (13). Overactivation of DNA replication will also lead to oncogene activation (14), increasing the incidence of tumors. Another remarkable characteristic of tumorigenesis is the disturbance of cell cycle regulatory mechanisms (15). P53 plays a key role in the G1 phase monitoring point of the human cell cycle, and mutations in this gene are present in more than 50% of tumors, including KIRC patients (16). Defects in the mismatch repair system are also closely associated with tumors, and mutations in any of the genes of this system can result in a functional defect, causing microsatellite instability leading to increased tumor incidence (17). Our results showed that high expression of NUF2 mRNA was associated with downregulation of the pathways of propanoate metabolism, pyruvate metabolism, the citrate cycle TCA cycle, and butanoate metabolism, which have been intrinsically linked to tumorigenesis and development (18). High expression of NUF2 mRNA in our study was also linked to the degradation of valine, leucine, and isoleucine. Downregulation of the degradation of branched-chain amino acids such as leucine and isoleucine can promote tumorigenesis and development through the PI3K/Akt/mTOR pathway (19). NUF2 was found important for the proliferation of colon cancer cells by cell cycle regulation (20). In Small Cell Esophageal Carcinoma, NUF2 was found to be linked with cell cycle, DNA repair and P53 pathway (21). However, the relationship between high expression of NUF2 mRNA in KIRC patients and the upregulation or downregulation of these pathways has not been investigated by researchers to date, and further experiments are needed to verify the mechanism of the role of NUF2 in the development of KIRC.
Our results show that NUF2 expression is associated with TNM staging, pathological grading, and the prognosis of KIRC patients, and that NUF2 can be used as an independent prognostic biomarker of KIRC. However, further studies are needed to identify its exact mechanisms of action. In addition, there are no NUF2 inhibitors available for clinical application, which should be one of the future research directions.
Acknowledgments
Funding: The authors acknowledge the financial grant by the Science and Technology Development Plan of Taicang in Jiangsu Province P.R.C. (grant numbers TC2020JCYL20).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-620
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tau-21-620
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-620). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First People’s Hospital of Taicang (NO. 2021-KY-161, the registration number of ethics board) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Liu D, Ding X, Du J, et al. Human NUF2 interacts with centromere-associated protein E and is essential for a stable spindle microtubule-kinetochore attachment. J Biol Chem 2007;282:21415-24. [Crossref] [PubMed]
- Jiang X, Jiang Y, Luo S, et al. Correlation of NUF2 Over-expression with Poorer Patient Survival in Multiple Cancers. Cancer Res Treat 2021; Epub ahead of print. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Mollica V, Di Nunno V, Massari F. Pembrolizumab plus axitinib: a new treatment option for patients with metastatic renal cell carcinoma. Chin Clin Oncol 2019;8:S21. [Crossref] [PubMed]
- Sansregret L, Vanhaesebroeck B, Swanton C. Determinants and clinical implications of chromosomal instability in cancer. Nat Rev Clin Oncol 2018;15:139-50. [Crossref] [PubMed]
- Obara W, Sato F, Takeda K, et al. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci 2017;108:1452-7. [Crossref] [PubMed]
- Sun J, Chen J, Wang Z, et al. Expression of NUF2 in breast cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao 2019;39:591-7. [PubMed]
- Hu P, Chen X, Sun J, et al. siRNA-mediated knockdown against NUF2 suppresses pancreatic cancer proliferation in vitro and in vivo. Biosci Rep 2015;35:00170. [Crossref] [PubMed]
- Huang SK, Qian JX, Yuan BQ, et al. SiRNA-mediated knockdown against NUF2 suppresses tumor growth and induces cell apoptosis in human glioma cells. Cell Mol Biol (Noisy-le-grand) 2014;60:30-6. [PubMed]
- Liu Q, Dai SJ, Li H, et al. Silencing of NUF2 inhibits tumor growth and induces apoptosis in human hepatocellular carcinomas. Asian Pac J Cancer Prev 2014;15:8623-9. [Crossref] [PubMed]
- Cimadamore A, Gasparrini S, Santoni M, et al. Biomarkers of aggressiveness in genitourinary tumors with emphasis on kidney, bladder, and prostate cancer. Expert Rev Mol Diagn 2018;18:645-55. [Crossref] [PubMed]
- Venkitaraman AR. Tumour suppressor mechanisms in the control of chromosome stability: insights from BRCA2. Mol Cells 2014;37:95-9. [Crossref] [PubMed]
- Ren L, Chen L, Wu W, et al. Potential biomarkers of DNA replication stress in cancer. Oncotarget 2017;8:36996-7008. [Crossref] [PubMed]
- Xu D, Li CF, Zhang X, et al. Skp2-macroH2A1-CDK8 axis orchestrates G2/M transition and tumorigenesis. Nat Commun 2015;6:6641. [Crossref] [PubMed]
- Harlander S, Schönenberger D, Toussaint NC, et al. Combined mutation in Vhl, Trp53 and Rb1 causes clear cell renal cell carcinoma in mice. Nat Med 2017;23:869-77. [Crossref] [PubMed]
- Li C, Liu F, Huang D, Wu Y, et al. The correlation between DNA mismatch repair status and the clinicopathological and molecular features of Chinese sporadic colorectal cancer. Transl Cancer Res 2020;9:137-44. [Crossref]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab 2016;23:27-47. [Crossref] [PubMed]
- Nie C, He T, Zhang W, et al. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int J Mol Sci 2018;19:954. [Crossref] [PubMed]
- Sugimasa H, Taniue K, Kurimoto A, et al. Heterogeneous nuclear ribonucleoprotein K upregulates the kinetochore complex component NUF2 and promotes the tumorigenicity of colon cancer cells. Biochem Biophys Res Commun 2015;459:29-35. [Crossref] [PubMed]
- Liu D, Xu X, Wen J, et al. Integrated Genome-Wide Analysis of Gene Expression and DNA Copy Number Variations Highlights Stem Cell-Related Pathways in Small Cell Esophageal Carcinoma. Stem Cells Int. 2018;2018:3481783 [Crossref] [PubMed]
(English Language Editor: B. Draper)









