
Efficacy and safety of aildenafil citrate in Chinese men with erectile dysfunction: a multicenter, randomized, double-blind, placebo-controlled crossover trial
Introduction
Erectile dysfunction (ED) refers to the persistent inability of the penis to achieve and/or maintain sufficient erections to have a satisfactory sexual life (1). It is a common sexual problem with a high incidence and prevalence all over the world (2,3). It has been reported that a quarter of newly diagnosed patients with ED were under the age of 40, and nearly 50% had severe symptoms (4). The etiology of ED is multifactorial and the underlying causes may be organic, physiologic, endocrine, and psychogenic factors (4,5). Orally-administrated phosphodiesterase type 5 (PDE5) inhibitors are commonly used for treating ED, mainly sildenafil, vardenafil, and tadalafil. These drugs have been beneficial in treating ED in a wide range of patients with varying etiologies of sexual dysfunction. The clinical management of ED has been rather static since the advent of PDE5 inhibitors, and new agents are required for those who do not respond to current treatment. Aildenafil citrate has been developed independently as a novel and potent PDE5 inhibitor in China for the treatment of ED, which significantly increases cGMP levels by inhibiting PDE5 activity to promote normal and hypoactive erectile function with sexual stimulation (Figure 1). The recommended dosage of aildenafil is 60 mg, which could be rapidly absorbed through the gastrointestinal tract (6). The plasma concentration of drug reaches the maximum in 60 min with a biological half-life of 4 hours. Aildenafil is metabolized mainly by cytochrome P4503A4 in liver microsomal enzymes, and most of its metabolites are excreted in the feces. The present trial aimed to investigate the efficacy and safety of single-dose aildenafil citrate in Chinese patients with mild to moderate ED. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/tau-21-441).
Methods
Study design
A multicenter, randomized, double-blind, placebo-controlled, crossover trial was performed involving patients with mild to moderate ED in three medical centers from March 2018 to June 2019. All the procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The trial was approved by the institutional review board of Peking University First Hospital (No. 2018005) and registered in the Chinese Clinical Trials Register (No. ChiCTR1900026025). Informed consent to participate in the study was provided before recruitment. The randomization sequence of the study was generated by an independent biostatistician, which was then imported into the interactive web response system. Eligible participants were assigned in a 1:1 ratio into the aildenafil group or placebo group. Patients then received aildenafil citrate (2 tablets, 60 mg, p.o.) or matching placebo in the order designed at randomization, with a 7-day washout period in between. Single-dose aildenafil citrate or placebo was administered orally on demand. Only the statistician who was independent of the trial and built the allocation sequence was aware of the study medication allocation sequence. Participants and all other personnel remained blinded until the study database lock.
Inclusion and exclusion criteria
The diagnosis of ED was made based on the Guidelines for the Diagnosis and Treatment of Erectile Dysfunction (2016 Edition). The inclusion and exclusion criteria were summarized in Table 1. According to the informed consent, the patients had the right to withdraw from the study throughout the journey, the reasons for which should be documented such as adverse events leading to treatment discontinuation, socioeconomic status, and no explanation.

Full table
Outcome measures
The primary outcome was the duration of penile rigidity over 60% of tip and base. Secondary outcomes were the duration of penile rigidity over 80% of tip and base, the time to onset of penile base rigidity over 60%, penile circumference, and penile erection hardness score (EHS). Patients received tablets and lied down in a supine position on a medical examination table. The RigiScan® Plus system (Gesiva Medical, Eden Prairie, MN, USA) was initiated and connected to the computer before the test. According to the manufacturer’s instructions, the tip and base tension guides were placed on the tip and base of the penis, respectively. The device automatically determined the baseline for penile circumference, rigidity and tumescence for the first 15 min. An audiovisual headset was placed on the participant’s head and adjusted to a comfortable volume. The visual sexual stimuli that lasted for 60 min were used to provoke an erectile response to complete a testing section, with each patient examined in a dark and silent room. Masturbation was prohibited during this session.
Safety outcomes included evaluation of vital signs and adverse events at baseline and each visit during treatment. Treatment-emergent adverse events (TEAEs) were recorded on the patient’s case report form. Clinical laboratory values, electrocardiogram readings, and vital signs were monitored throughout the trial. Commonly reported TEAEs were coded using the medical dictionary for regulatory activities preferred term.
Statistical analysis
The sample size was calculated using PASS 13 software (NCSS, LLC. Kaysville, Utah, USA). We calculated that the sample size of 22 per group would provide 80% power at the 5% (two sides) alpha level. Finally, 60 subjects were randomized in a 1:1 ratio to aildenafil or placebo groups (30 in each group), assuming the rate of dropouts. We performed primary analysis according to the intention-to-treat principle, which included all patients randomly assigned to the study. The secondary per-protocol analyses were performed among randomized patients who adhered to the protocol without any major deviations. Safety analyses were performed to evaluate all randomly assigned patients who received at least one dose of aildenafil.
Continuous variables were demonstrated as mean ± standard deviation or the median value (IQR: 25th, 75th). The continuous variables between the two groups were compared using Student’s t-test or Wilcoxon rank-sum test. Categorical variables were described as frequencies and percentages and the comparisons were done with the chi-square test or Fisher’s exact test. The covariance model was used for adjustment of treatment-by-center interaction and dosing order. Statistical tests were performed using the SAS® software, version 9.4 (SAS Institute, Cary, NC, USA). P<0.05 were considered to indicate statistical significance.
Results
Patient demographics
Of the 61 patients screened for this study, 60 were randomly assigned into the placebo group (n=30) and the aildenafil group (n=30) from March 2018 to June 2019. After two cycles of administration, 57 of them completed the trial (30 in the aildenafil group and 27 in the placebo group) (Figure 2). Inclusion or exclusion criteria were demonstrated in Table 1. Clinical characteristics and baseline demographics for the randomized population were shown in Table 2. Baseline characteristics were well balanced across groups without statistically significant difference (P>0.05 for all).
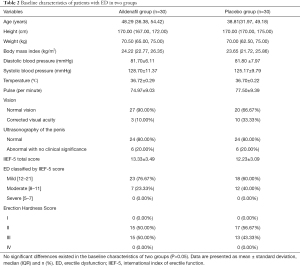
Full table
Efficacy
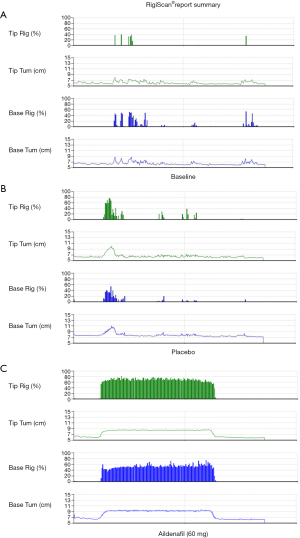
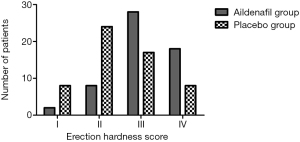
Aildenafil citrate demonstrated obvious improvements in erectile function as measured by the RigiScan® Plus system (Figure 3). The duration of penile rigidity over 60% of tip and base in the aildenafil group was significantly increased compared with the placebo group (P<0.001 for both) (Table 3). After the adjustment of treatment-by-center interaction and dosing order, the erectile function was improved followed by the oral administration of aildenafil (P<0.001) (Table 4). The duration of penile tip rigidity over 80% did not differ significantly between the two groups (P=0.077). Nonetheless, the duration of penile base rigidity over 80% was significantly increased in the aildenafil group versus the placebo group (P=0.004). The time to onset of penile rigidity over 60% was not statistically significant between the two groups (P=0.250). Moreover, the penile circumference of two groups was similar at full erection and flaccid state. The erectile function measured by EHS was significantly improved in the aildenafil group compared with the placebo group (P<0.001) (Figure 4).

Full table

Full table
Safety
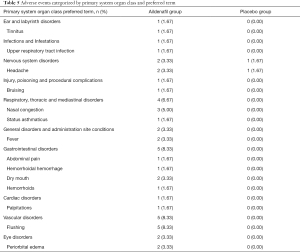
A total of 24 adverse events were reported from 14 patients in the aildenafil group including 19 TEAEs from 10 patients, as compared with one in the placebo group (Table 5). However, most of them were mild-moderate and none withdrew from the study due to TEAEs. Severe TEAEs or death related to TEAEs did not occur in both groups during the trial. The most common TEAEs in the study were flushing sensation (8.33%), nasal congestion (5.00%), and headache (3.33%). Abnormal results with clinical significance were not observed from laboratory findings, electrocardiogram readings, and vital signs.
Full table
Discussion
Considered as a common type of male sexual dysfunction, ED is characterized by the regular or repeated inability to achieve or maintain an erection of sufficient rigidity to accomplish sexual activity. Owing to failing to have satisfactory sexual performance, the patients may have negative self-image and problems in sexual relationships that can cause psychological harm. ED was considered, in most cases, to be a purely psychogenic disorder, but current evidence suggests that more than 80% of cases have an organic etiology, including endocrine, vasculogenic, neurogenic, and iatrogenic (2). Oral phosphodiesterase inhibitors are the first-line nonsurgical interventions for the management of ED. Mechanistically, these drugs competitively inhibit PDE5, leading to a build-up of cGMP upon the secretion of nitric oxide and initiating a cascade of events that lead to smooth muscle relaxation (7). Aildenafil citrate is a novel and selective inhibitor of cyclic guanosine monophosphate cGMP-specific PDE5 and the predominant isozyme metabolizing cGMP in the corpus cavernosum (8). Preclinical assessments demonstrated that aildenafil citrate can significantly shorten the time to onset of erections as well as increase the number of erectile responses and the duration of erection (9). In the present study, we evaluated the efficiency and safety of single-dose aildenafil citrate in patients with ED, as measured by RigiScan® Plus and EHS.
To evaluate the efficacy of aildenafil citrate in male patients with mild to moderate ED, RigiScan® Plus combined with visual sexual simulation was used in this study to continuously detect the erectile status after medication. RigiScan® Plus has been adopted to measure erectile functions as a precision diagnostic instrument for decades (10,11). The International Index of Erectile Function (IIEF) is a widely used, multi-dimensional patient questionnaire for the assessment of male sexual function, which has been recommended for diagnostic evaluation of ED severity (12,13). However, several disadvantages of its measurement properties have also been shown such as inconsistent structural validity and test-retest reliability. In this study, a single dose of aildenafil (60 mg) in heterosexual men with mild to severe ED showed efficiency when compared with placebo for all primary outcomes. As for secondary outcomes, the erectile functions improved by oral administration of aildenafil were not consistent. The duration of penile base rigidity over 80% was significantly increased, whereas the duration of penile tip remains unchanged. Moreover, findings based on the time to onset of penile rigidity over 60% did not demonstrate a rapid penile response to sexual stimulation. However, EHS was relatively improved in the aildenafil group, indicating that aildenafil is effective for treating sexual dysfunction. As the first oral treatment approved to treat ED, sildenafil has been shown to improve erections, leading to successful intercourse in 63% of men with general ED compared with 29% of men using a placebo (14). The onset driven by sildenafil is typically within 20 minutes and lasts for about 2 hours. Though not significantly different from placebo, the onset driven by aildenafil was within approximately 15 min. Given the improved efficiency, aildenafil has the potential to provide an additional option in the treatment of ED.
The most commonly reported TEAEs seen in the aildenafil groups were generally consistent with those observed with other PDE5 inhibitors (15-17). In this trial, safety analyses showed that the incidence of TEAEs related to aildenafil citrate was low and mild, and no severe adverse events leading to withdrawal occurred. During medical consultation, patients should be informed of the possible adverse events of these drugs, which may include headache, dizziness, facial flushing, nasal congestion, and visual disturbances.
Our study has several limitations worth noting. First, patients with ED originating from type 1 or type 2 diabetes or those with other sexual dysfunctions were excluded, which may restrict the indications. Second, the long-term efficiency of aildenafil was not evaluated to determine whether drug resistance occurred. Third, the IIEF and the quality of life that represented the patient’s experience and satisfaction were not measured in the trial. A combination of IIEF and RigiScan® Plus may be more effective in the evaluation of disease severity. Further research is warranted to better assess the role of aildenafil in the treatment of ED.
Conclusions
These results suggested that aildenafil 60 mg, especially in the on-demand treatment, is safe and effective in treating patients with mild to moderate ED. Aildenafil has been well-tolerated with a low incidence of TEAEs, which may function as a novel potential PDE5 inhibitor in the management of ED.
Acknowledgments
The authors would like to thank the patients who enrolled in the study, the contributions of health professionals, Lei Yang from Youcare Pharmaceutical Group for coordination, Run-Yan Wang for statistical analysis, and Dr. Xiao-Hui Tan for language editing.
Funding: Funded by Youcare Pharmaceutical Group.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-441
Trial Protocol: Available at https://dx.doi.org/10.21037/tau-21-441
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tau-21-441
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-441). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). The trial was approved by the institutional review board of Peking University First Hospital (No. 2018005) and registered in the Chinese Clinical Trials Register (No. ChiCTR1900026025). Informed consent to participate in the study was provided before recruitment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCabe MP, Sharlip ID, Atalla E, et al. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J Sex Med 2016;13:135-43. [Crossref] [PubMed]
- Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010;57:804-14. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010;7:1598-607. [Crossref] [PubMed]
- Ma WG, Jia JM. The effects and prospects of the integration of traditional Chinese medicine and Western medicine on andrology in China. Asian J Androl 2011;13:592-5. [Crossref] [PubMed]
- He ZJ, Zhang K, Jin J, et al. Aildenafil citrate: a new potent and highly selective phosphodiesterase type 5 inhibitor for the treatment of erectile dysfunction. Zhonghua Nan Ke Xue 2006;12:1080-3. [PubMed]
- Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802-13. [Crossref] [PubMed]
- Wang J, Jiang Y, Wang Y, et al. Liquid chromatography tandem mass spectrometry assay to determine the pharmacokinetics of aildenafil in human plasma. J Pharm Biomed Anal 2007;44:231-5. [Crossref] [PubMed]
- Wang W, Zhao Z, He X, et al. Effects of Aildenafil on Penile Erection. Chinese Journal of Pharmacology and Toxicology 2005;3:220-5.
- Elhanbly S, Elkholy A. Nocturnal penile erections: the role of RigiScan in the diagnosis of vascular erectile dysfunction. J Sex Med 2012;9:3219-26. [Crossref] [PubMed]
- Jannini EA, Granata AM, Hatzimouratidis K, et al. Use and abuse of Rigiscan in the diagnosis of erectile dysfunction. J Sex Med 2009;6:1820-9. [Crossref] [PubMed]
- Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers 2016;2:16003. [Crossref] [PubMed]
- Neijenhuijs KI, Holtmaat K, Aaronson NK, et al. The International Index of Erectile Function (IIEF)-A Systematic Review of Measurement Properties. J Sex Med 2019;16:1078-91. [Crossref] [PubMed]
- Padma-Nathan H. Sildenafil citrate (Viagra) treatment for erectile dysfunction: An updated profile of response and effectiveness. Int J Impot Res 2006;18:423-31. [Crossref] [PubMed]
- Cui YS, Li N, Zong HT, et al. Avanafil for male erectile dysfunction: a systematic review and meta-analysis. Asian J Androl 2014;16:472-7. [Crossref] [PubMed]
- Porst H, Padma-Nathan H, Giuliano F, et al. Efficacy of Tadalafil for the Treatment of Erectile Dysfunction at 24 and 36 Hours After Dosing: A Randomized Controlled Trial. Urology 2003;62:121-5; discussion 125-6. [Crossref] [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404. [Crossref] [PubMed]




