Current guidelines in the management of interstitial cystitis
IntroductionOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
Interstitial cystitis (IC), also referred to as bladder pain syndrome (BPS), is a heterogeneous chronic disease of unknown etiology that impacts up to 8 million women in America (1). Patients suffer from pelvic pain that can be exacerbated by bladder filling, and is often associated with urinary frequency and urgency (2). Since Alexander Skene’s inception of the term in 1887, research into the etiology and pathophysiology of this disease has not been successful in elucidating a specific mechanism, revealing more questions than answers. As a result, treatment is difficult.
The American Urological Association (AUA) breaks down the treatment recommendations into six tiers of treatment with the fundamental principle of using more conservative therapies first, with less conservative therapies employed if symptom control is inadequate for acceptable quality of life (Table 1) (3). These treatment guidelines begin with the simple clinical principles of education and lifestyle modifications and progress through levels of physical, pharmacological, and ultimately surgical therapies for those that fail the less invasive therapies.

Full table
The purpose of this review is to outline the recommendations for the treatment of IC and the evidence from which these recommendations arise.
Conservative therapyOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
As with most diseases first line therapy for IC is conservative management with techniques including education, behavioral modification, and stress control. Patient education as to the normal function of the bladder as well as what behaviors may lead to increased bladder pain is integral to symptom control. Behavioral modification strategies may include: altering diet to avoid known bladder irritants such as caffeine and spicy foods, altering urine volume to control concentration, application of local heat or cold over the bladder or perineum and exercises that improve pelvic floor muscle relaxation and bladder training. IC flairs are also often associated with psychological stress, so stress reduction techniques such as meditation also play an important role in symptom control.
Beyond lifestyle changes, physical therapy is also playing an increasing role in the treatment of IC. Second line treatment begins with physical therapy, and the AUA recently upgraded appropriate manual physical therapy techniques that resolve abdominopelvic muscular trigger points and improve connective tissue restrictions as a standard of care with grade A evidence (4). These recommendations are based on a randomized clinical trial by Fitzgerald and colleagues that tested ten 60-minute sessions over 12 weeks of myofascial physical therapy (MPT) compared to global therapeutic massage (GTM) in IC patients. At 3 months, significantly more patients in the MPT group reported moderate or marked improvement compared to GTM group (59% versus 26%) (5). It should also be noted that these therapies are not pelvic floor strengthening therapy, and that such exercises may actually worsen IC symptoms (4).
Pharmacological therapyOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
Oral therapy
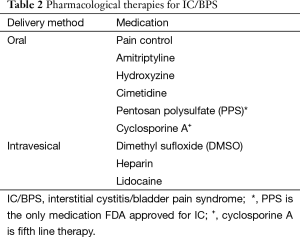
For those patients that fail conservative therapy, oral medications are the appropriate next line of treatment. Table 2 demonstrates the currently accepted pharmacological therapies for IC. Introduction of pharmacological strategies should be done in parallel with continued conservative therapies and should involve pain control in addition to disease modifying agents (3). In terms of pain management, the principles for IC treatment should be similar to those for management of other chronic pain states. Beyond pain control, the following medications are recommended by the AUA.
Full table
Amitriptyline
Amitriptyline is a tricyclic antidepressant and has been demonstrated to be effective for various causes of neuropathic pain. One randomized controlled trial reported efficacy of oral amitriptyline to be superior to placebo with 63% of treatment group clinically significantly improved at 4 months versus 4% of placebo group (6). These benefits, however, must be weighed against adverse events. Although not life-threatening, observational studies report up to a 79% adverse reaction rate with side effects including nausea, drowsiness, weight gain and sedation (7). For those patients that do not tolerate amitriptyline other neurological modifying agents such as gabapentin, pregabalin or serotonin-norepinephrine reuptake inhibitors like milnacipran and duloxetine can be substituted, though these treatments are less well studied.
Hydroxyzine/cimetidine
Hydroxyzine and cimetidine are an H1-receptor agonist and an H2-receptor antagonist, respectively. These drugs may affect IC by preventing mast cell degranulation and histamine release (one of the mechanisms that has been suggested in the pathophysiology of IC) (8). While both drugs have multiple observational studies that suggest their effectiveness, each only has one randomized clinical trial. For cimetidine, Thilagarajah and colleagues demonstrated a statistically significant improvement in patient symptoms after 3 months of treatment (9). For hydroxyzine, however, the only available randomized clinical trial demonstrated clinical, but not statistically significant improvement (10). As such the recommendation for cimetidine is grade B and the recommendation for hydroxyzine is grade C.
Pentosan polysulfate (PPS)
PPS is a polysulfated xylan and the only FDA-approved oral medication to treat IC (8). This medication is thought to exert its effect by repairing the glycosaminoglycan (GAG) layer of the bladder urothelium and reducing its permeability (11). PPS holds a grade B recommendation based on five clinical trials (four of which were randomized clinical trials). Of these trials, two demonstrated significant symptom improvement with PPS (12,13), while the other two did not (10,14). However, since publication of the guidelines update another randomized clinical trial by Nickel and colleagues found no difference between PPS (with two separate arms with different dosing regimens) and placebo (15), so the next iteration of the guidelines may see a change in the recommendations regarding this drug.
Cyclosporine A (CyA)
Unlike the other oral medications, CyA is actually a fifth line therapy and reserved for refractory patients. CyA inhibits calcineurin, which is necessary for the activation of T cells. As such it is generally used for immunomodulation in transplant recipients and certain autoimmune disorders. Given this mechanism of action it has also been proposed that this drug may benefit patients with bladder inflammation caused by IC.
The evidence for the use of CyA comes from one randomized control trial and four observational studies. For the randomized control trial Sairanen and colleagues randomized 64 IC patients to either CyA or PPS treatment over 6 months. What they found was that 75% of patients in the CyA group experienced clinically significant improvement compared to 19% of a PPS comparison group after the allotted time frame (16). The observational studies found similar efficacy, with particular efficacy noted in patients with active inflammation or Hunner’s lesions (16-19). However, because of the relatively small numbers represented in these studies, the lack of long-term follow-up data, and the potential for serious side effects, the AUA lists CyA as an option with grade C evidence. They also recommend that, should this option be chosen, it only be done so by clinicians with significant experience dosing CyA (3).
Intravesicular therapyOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
Bladder instillation therapy refers to the direct introduction of a treatment agent into the bladder via a catheter (20). Although still considered second line therapy, these treatments are reserved for patients who fail conservative management as well as oral medications. Table 2 shows the most commonly used intravesicular therapies.
Dimethyl sulfoxide (DMSO)
DMSO is an organosulfur compound with chemical formula (CH3)2SO. Although the exact mechanism by which DMSO alleviates IC is unknown, it is believed to work through several mechanisms: studies have suggested that it may act by reducing inflammation, causing detrusor relaxation, or dissolving collagen, as well as by acting as an analgesic (21). DMSO may also cause temporary urothelial injury (22) and therefore may allow for better penetration of other agents. As such, DMSO is often given as part of a “cocktail” in a multimodal regimen (23). These cocktails include some combination of DMSO, heparin, lidocaine, sodium bicarbonate, and/or steroid, but no combinations have been proven more effective than others. The general DMSO only regimen involves initial 50 mL doses weekly for 6-8 weeks, followed by 50 mL maintenance doses every 2 weeks for 3-12 months (21).
Heparin and GAGs
Heparin is the highly sulfonated GAG best known for its use as an anticoagulant. In addition to its hematological uses, heparin has also been adopted as an intravesicular treatment for IC. The theoretical benefit of heparin in these patients is derived from the histopathogical changes exhibited in IC: when diseased urothelium exhibits a loss of its endogenous proteoglycans (24,25). Like oral PPS, heparin has the potential to act as an exogenous GAG, and may be able to replace some of the urothelium’s natural function (26,27). Heparin also demonstrates a variety of other potentially beneficial effects, including anti-inflammatory, fibroblast proliferation inhibition, angiogenesis, and smooth muscle cell proliferation, and therefore may act in IC by multiple mechanisms (28).
With the success of heparin, other GAGs have also been considered for use in treating IC, but none have proven successful. The most common is the nonsulfonated GAG hyaluronic acid. Although it sees some use in Europe and Canada, hyaluronic acid has not been proven to be effective in any randomized trials. The drug actually underwent two multi-center, double-blinded, placebo-controlled industry funded studies in 2003 and 2004, and neither demonstrated significant efficacy (29). Over the counter supplements containing the GAGs chondroitin and the GAG precursor glucosamine are available, but have never been subjected to randomized clinical trials.
Lidocaine
Lidocaine is a common topical anesthetic that has been used in a wide variety of pain syndromes, and the use of such anesthetics has long been practiced in the treatment of IC. It is given in a wide array of different formulations and concentrations, and recently has seen increasing use in combination with an alkalizing agent in order to avoid ionization within the acidic urine and better penetrate urothelium (30). Unfortunately, the relief granted by lidocaine is rarely long lasting (longer than 2 weeks). Currently researchers are attempting to remedy this problem with implantable lidocaine eluding devices, and initial results are positive (31).
Surgical therapyOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
For patients that continue to fail symptom control with a full course of pharmacological treatments more invasive treatment may be necessary. While the most extreme cases of IC may require removal or diversion from the bladder (sixth line therapy), the vast majority of surgical therapies are more benign.
Cystoscopy with hydrodistention/fulguration of lesions
If conservative and pharmacological therapies have not provided acceptable symptom control, then third line therapy is to perform cystoscopy under anesthesia with low-pressure (60 to 80 cmH2O), short duration (less than 10 minutes) hydrodistension. This procedure has multiple benefits. First and foremost it serves as a diagnostic tool by allowing the clinician to inspect the bladder for glomerulations and Hunner’s lesions or any other bladder lesions. It also allows for staging by measuring anatomical capacity (rather than just functional capacity). Beyond the diagnostic properties of cystoscopy, hydrodistention is in itself a treatment for IC. Although the exact mechanism of action by which hydrodistention relieves pain is unclear, it has been theorized that hydrodistention allows for the break down and subsequent reconstruction of damaged nerve pathways, even in the absence of lesions (32). Regardless of the mechanism, hydrodistention has been demonstrated in multiple studies to be safe with relatively low rates of adverse events and to effectively relieve pain for up to 6 months (33,34).
Another important aspect to cystoscopy is that it allows for the examination of the bladder for Hunner’s lesions. These lesions present as a reddened mucosal area with small vessels radiating towards a central scar, and have increased likelihood of bleeding, especially during hydrodistention. Although relatively rare (occurring in only 5-10% of patients), identification of Hunner’s lesions is important because treatment appears to constitute one of the few IC therapies that results in significant prolonged improvement with only a single exposure to the procedure. Fulguration or sclerosing of Hunner’s lesions has been demonstrated multiple times to result in complete or almost complete resolution of pain symptoms for durations of over a year (35,36). Intralesional injection of corticosteroids such as triamcinolone is also useful in reducing symptoms, though safety data on the maximum dose of such injections is lacking.
Intradetrusor botulinum toxin A (BTX-A)
Fourth line treatments for IC include intradetrusor BTX-A injections and neuromodulation. Botulinum toxin is a neurotoxic protein produced by the bacterium Clostridium botulinum. Although it occurs in sever subtypes (A-G) in nature, in medical practice the only subtypes used are BTX-A and less commonly BTX-B. BTX-A has long been considered an efficacious treatment for various bladder pathologies such as overactive bladder (OAB) and neurogenic bladder, but is only recently being explored for the treatment of IC. For the treatment of IC current AUA guidelines list BTX-A as an option with grade C evidence. This option is based upon one randomized control trial and nine observational studies that demonstrated highly variable amounts of short and long efficacy rates (from 20% to 86% at 3 months) (37-41).
Given the novelty of using BTX-A for IC, there is still some debate as to the best strategy for treatment. The one randomized control trial for BTX-A in IC patients found significant improvement in patients who undergo hydrodistention with BTX-A than those who undergo hydrodistention alone: successful treatment response at 12 and 24 months was reported in 24 (55%) and 13 (30%) patients in BTX-A group, respectively, compared with only six (26%) and four (17%) in the hydrodistention only group (42). This suggests that BTX-A is best given in conjunction with hydrodistention, but more evidence is required to formulate the optimal regimen.
Neuromodulation
Neuromodulation through sacral nerve stimulation (SNS) involves an initial test phase with insertion of a test lead tunneled under the skin transmitted onto the nerve roots exiting the S3 foramen. The pelvic and pudendal nerves are stimulated by an external stimulator that is later exchanged for a permanent implant if successful. This technique has been shown to be effective in several disease states including fecal incontinence, urinary urgency, urge incontinence and urinary retention and has an FDA indication for these symptoms. Early studies investigating the efficacy of neuromodulation on pain secondary to IC have also been positive, but the research is scant and highly variable. Thus, patients must be advised that neuromodulation is to be used for the treatment of voiding symptoms and that pain relief may not occur.
ConclusionsOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
Although the pathophysiology of IC is still poorly understood, a wide number of treatment options are available depending on severity of symptoms. As we better understand the disease process customized treatment should improve outcomes. Until personalized treatments become available application of the AUA guidelines is the best strategy to find the most beneficial and least invasive treatments for our patients in a timely fashion.
AcknowledgementsOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
None.
FootnoteOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Introduction
- Conservative therapy
- Pharmacological therapy
- Intravesicular therapy
- Surgical therapy
- Conclusions
- Acknowledgements
- Footnote
- References
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540-4. [PubMed]
- Wein AJ, Kavoussi LR, et al, editors. Campbell-Walsh Urology. 10th Edition. Philadelphia: Elsevier Saunders, 2012.
- Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162-70. [PubMed]
- Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193:1545-53. [PubMed]
- FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol 2012;187:2113-8. [PubMed]
- van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol 2004;172:533-6. [PubMed]
- van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol 2005;174:1837-40. [PubMed]
- Barr S. Diagnosis and management of interstitial cystitis. Obstet Gynecol Clin North Am 2014;41:397-407. [PubMed]
- Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int 2001;87:207-12. [PubMed]
- Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol 2003;170:810-5. [PubMed]
- Parsons CL. The therapeutic role of sulfated polysaccharides in the urinary bladder. Urol Clin North Am 1994;21:93-100. [PubMed]
- Mulholland SG, Hanno P, Parsons CL, et al. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology 1990;35:552-8. [PubMed]
- Parsons CL, Benson G, Childs SJ, et al. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol 1993;150:845-8. [PubMed]
- Holm-Bentzen M, Jacobsen F, Nerstrøm B, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urol 1987;138:503-7. [PubMed]
- Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. J Urol 2015;193:857-62. [PubMed]
- Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol 2005;174:2235-8. [PubMed]
- Forsell T, Ruutu M, Isoniemi H, et al. Cyclosporine in severe interstitial cystitis. J Urol 1996;155:1591-3. [PubMed]
- Sairanen J, Forsell T, Ruutu M. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol 2004;171:2138-41. [PubMed]
- Ehrén I, Hallén Grufman K, Vrba M, et al. Nitric oxide as a marker for evaluation of treatment effect of cyclosporine A in patients with bladder pain syndrome/interstitial cystitis type 3C. Scand J Urol 2013;47:503-8. [PubMed]
- Colaco MA, Evans RJ. Current recommendations for bladder instillation therapy in the treatment of interstitial cystitis/bladder pain syndrome. Curr Urol Rep 2013;14:442-7. [PubMed]
- Moldwin RM, Evans RJ, Stanford EJ, et al. Rational approaches to the treatment of patients with interstitial cystitis. Urology 2007;69:73-81. [PubMed]
- Hohlbrugger G, Lentsch P. Intravesical ions, osmolality and pH influence the volume pressure response in the normal rat bladder, and this is more pronounced after DMSO exposure. Eur Urol 1985;11:127-30. [PubMed]
- Stav K, Beberashvili I, Lindner A, et al. Predictors of response to intravesical dimethyl-sulfoxide cocktail in patients with interstitial cystitis. Urology 2012;80:61-5. [PubMed]
- Slobodov G, Feloney M, Gran C, et al. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol 2004;171:1554-8. [PubMed]
- Hurst RE, Roy JB, Min KW, et al. A deficit of chondroitin sulfate proteoglycans on the bladder uroepithelium in interstitial cystitis. Urology 1996;48:817-21. [PubMed]
- Hanno PM, Fritz R, Wein AJ, et al. Heparin as antibacterial agent in rabbit bladder. Urology 1978;12:411-5. [PubMed]
- Kyker KD, Coffman J, Hurst RE. Exogenous glycosaminoglycans coat damaged bladder surfaces in experimentally damaged mouse bladder. BMC Urol 2005;5:4. [PubMed]
- Lane DA, Adams L. Non-anticoagulant uses of heparin. N Engl J Med 1993;329:129-30. [PubMed]
- Reece JB, Urry L, Cain M, et al. Campbell biology. 9th edition; International edition. Harlow: Pearson Education, 2011.
- Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int 2009;103:910-8. [PubMed]
- Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Sci Transl Med 2012;4:143ra100.
- Niimi A, Nomiya A, Yamada Y, et al. Hydrodistension with or without fulguration of hunner lesions for interstitial cystitis: Long-term outcomes and prognostic predictors. Neurourol Urodyn 2015. [Epub ahead of print]. [PubMed]
- Hsieh CH, Chang WC, Huang MC, et al. Hydrodistention plus bladder training versus hydrodistention for the treatment of interstitial cystitis. Taiwan J Obstet Gynecol 2012;51:591-5. [PubMed]
- Aihara K, Hirayama A, Tanaka N, et al. Hydrodistension under local anesthesia for patients with suspected painful bladder syndrome/interstitial cystitis: safety, diagnostic potential and therapeutic efficacy. Int J Urol 2009;16:947-52. [PubMed]
- Payne RA, O'Connor RC, Kressin M, et al. Endoscopic ablation of Hunner's lesions in interstitial cystitis patients. Can Urol Assoc J 2009;3:473-7. [PubMed]
- Hillelsohn JH, Rais-Bahrami S, Friedlander JI, et al. Fulguration for Hunner ulcers: long-term clinical outcomes. J Urol 2012;188:2238-41. [PubMed]
- Smith CP, Radziszewski P, Borkowski A, et al. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology 2004;64:871-5; discussion 875. [PubMed]
- Kuo HC. Preliminary results of suburothelial injection of botulinum a toxin in the treatment of chronic interstitial cystitis. Urol Int 2005;75:170-4. [PubMed]
- Giannantoni A, Costantini E, Di Stasi SM, et al. Botulinum A toxin intravesical injections in the treatment of painful bladder syndrome: a pilot study. Eur Urol 2006;49:704-9. [PubMed]
- Ramsay AK, Small DR, Conn IG. Intravesical botulinum toxin type A in chronic interstitial cystitis: results of a pilot study. Surgeon 2007;5:331-3. [PubMed]
- Liu HT, Tyagi P, Chancellor MB, et al. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int 2009;104:1476-81. [PubMed]
- Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int 2009;104:657-61. [PubMed]


